Internally Functionalized Dendrimers
Carborane-containing Dendrimers - Carboranes are icosahedral clusters with molecular formula C2B10H12 that have attracted a great deal of attention for more than 40 years (see structures below). The chemistry of these clusters has been widely explored, and a number of different carborane containing molecules, ranging from biomolecules to polymers, have been reported. Perhaps the most interesting aspect of carboranes, and other boron-rich compounds, is that upon irradiation with thermal (slow) neutrons, a nuclear fission reaction results, breaking boron-10 atoms (which have 20% natural abundance) into an alpha particle, a lithium atom, and some gamma radiation. It was found in the 1930's that the ionizing radiation produced by this reaction has a penetration length in biological tissues of approx-imately 10 microns, or one human cell diameter. In 1936, Locher proposed that neutron irradiation of 10B delivered to malignant cells could be used to treat diseases such as cancer, and this treatment was dubbed Boron Neutron Capture Therapy (BNCT). More recently, a similar approach has been investigated for the treatment of refractory cases of rheumatoid arthritis (RA), in which the neutron capture reaction is used to destroy the inflamed synovial lining of a joint that causes debilitating pain to the patient. This process is termed Boron Neutron Capture Synovectomy (BNCS). Despite the great potential of BNCT and BNCS in treating disease, clinical success with both of these therapies has been limited. The main challenge to these approaches has been the delivery of adequate amounts of boron (10-30 g of 10B per g of tumour) to the site of interest, and especially into the cells that are to be destroyed. Carboranes, with their high B content have been the molecules of choice for B-delivery, but are themselves inadequate due to their hydrophobicity. Therefore, vehicles for delivery of carboranes to disease tissues are critical to the success of BNCT and BNCS.
Dendrimers are a class of polymers that are precisely synthesized, monodisperse, and can exhibit a large number of functionalizable end-groups. This multivalency of dendrimers makes them attractive scaffolds for drug delivery applications, as a high loading of pharmacophores can be achieved on each structure. In addition, if the pharmacophore is incorporated within the interior of the dendrimer, the periphery of the macromolecule can be tailored for solubility and specific targeting functions. We have recently reported the preparation of a new class of aliphatic polyester dendrimers that can serve as potential carborane carriers. These structures are promising because similar dendrimers have previously been shown to be water-soluble, biocompatible, non-toxic, and biodegradable.
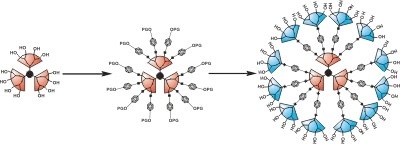
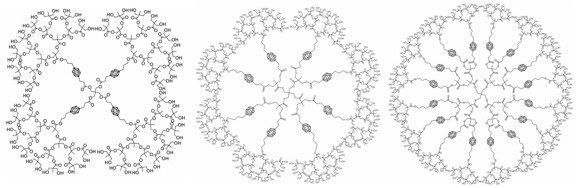
Our approach involves the internal functionalization of these polyester dendrimers with a controllable number of carborane cages, while retaining water solubility because of a large number of hydroxyl groups on their periphery. Below is a schematic representation of the synthetic strategy, where an initial dendritic core is peripherally functionalized with a bifunctional carborane synthon that can be subsequently deprotected to allow for further dendronization. The largest water-soluble structures we have synthesized thus far are also depicted below. Currently, we are working toward determining the biodistribution and efficacy of these molecules, as well as analogous polymeric species.
Dendrimers are a class of polymers that are precisely synthesized, monodisperse, and can exhibit a large number of functionalizable end-groups. This multivalency of dendrimers makes them attractive scaffolds for drug delivery applications, as a high loading of pharmacophores can be achieved on each structure. In addition, if the pharmacophore is incorporated within the interior of the dendrimer, the periphery of the macromolecule can be tailored for solubility and specific targeting functions. We have recently reported the preparation of a new class of aliphatic polyester dendrimers that can serve as potential carborane carriers. These structures are promising because similar dendrimers have previously been shown to be water-soluble, biocompatible, non-toxic, and biodegradable.
Our approach involves the internal functionalization of these polyester dendrimers with a controllable number of carborane cages, while retaining water solubility because of a large number of hydroxyl groups on their periphery. Below is a schematic representation of the synthetic strategy, where an initial dendritic core is peripherally functionalized with a bifunctional carborane synthon that can be subsequently deprotected to allow for further dendronization. The largest water-soluble structures we have synthesized thus far are also depicted below. Currently, we are working toward determining the biodistribution and efficacy of these molecules, as well as analogous polymeric species.
Key References: J. Am. Chem. Soc., 2005, 127, 12081-12087; Langmuir, 2006, 22, 5251-5255.
