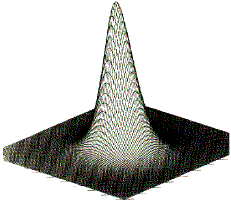
Figure 1. (a) The electron density in the plane containing the two carbon and four hydrogen nuclei of the ethene molecule, portrayed as a projection in the third dimension and in the form of a contour map. The absolute maxima in ![]() (r) attained at the positions of the carbon nuclei are not shown because of their large values.
(r) attained at the positions of the carbon nuclei are not shown because of their large values.

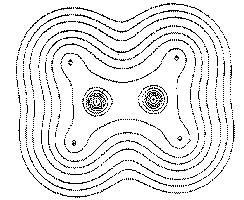
Figure 1. (b) Same as in Figure 1a, but for a plane obFigure 1. (b) Same as in Figure 1a, but for a plane obtained by a rotation of 90° about the C-C axis, a plane containing only the carbon nuclei.

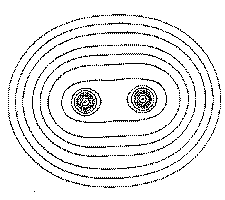
Figure 1. (c) Again, the same portrayal as in Figure 1a, but this time for a plane perpendicular to the C-C axis at its mid-point. What appears as a C-C saddle in (a) is seen to be a maximum in the plane perpendicular to the C-C axis. The point exhibits two negative curvatures perpendicular to this axis and one positive curvature along the axis.

Return to Theory of Atoms in Molecules: "What is a Bond?"
Go to: Richard Bader's Home Page | Chemistry Faculty | Chemistry Welcome Page.
Last update: 23feb96, rfwb
"));