 |
Richard F.W. BaderOctober 15, 1931 - January 15, 2012 B.Sc., M.Sc. (McMaster), Ph.D. (M.I.T.), F.R.S.C., F.C.I.C. Theoretical Investigations of Molecular Structure and Reactivity |
Obituaries & Tributes:
Dr. Bader's lecture on "There Are No Bonds - Only Bonding!"delivered in
the ‘Frontiers in Chemistry Series’ at
Case Western Reserve University, January 31, 2008.
The molecular structure hypothesis, that a molecule is a collection of atoms linked by a network of bonds - was forged in the crucible of nineteenth century experimental chemistry. It has continued to serve as the principal means of ordering and classifying the observations of chemistry. The difficulty with this hypothesis was that it was not related directly to quantum mechanics, the physics which governs the motions of the nuclei and electrons that make up the atoms and the bonds. Indeed there was, and with some there still is, a prevailing opinion that these fundamental concepts, while unquestionably useful, were beyond theoretical definition. We have in chemistry an understanding based on a classification scheme that is both powerful and at the same time, because of its empirical nature, limited.
Richard Feynman and Julian Schwinger have given us a reformulation of physics that enables one to pose and answer the questions "what is an atom in a molecule and how does one predict its properties?" These questions were posed in my laboratory where it was demonstrated that this new formulation of physics when applied to the observed topology of the distribution of electronic charge in real space, yields a unique partitioning of some total system into a set of bounded spatial regions. The form and properties of the groups so defined faithfully recover the characteristics ascribed to the atoms and functional groups of chemistry. By establishing this association, the molecular structure hypothesis is freed from its empirical constraints and the full predictive power of quantum mechanics can be incorporated into the resulting theory - a theory of atoms in molecules and crystals.
The theory recovers the central operational concepts of the molecular structure hypothesis, that of a functional grouping of atoms with an additive and characteristic set of properties, together with a definition of the bonds that link the atoms and impart the structure. Not only does the theory thereby quantify and provide the physical understanding of the existing concepts of chemistry, it makes possible new applications of theory. These new applications will eventually enable one to perform on a computer, in a manner directly paralleling experiment, everything that can now be done in the laboratory, but more quickly and more efficiently, by linking together the functional groups of theory, such as the one illustrated in Figure 1. These applications include the design and synthesis of new molecules and new materials with specific desirable properties.
A scientific biography of R. F. W. Bader for Grade XII
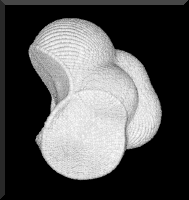
Figure 1. The serinyl group |NHCH(CH2OH)C(=O)|, a fragment of a polypeptide. The topology of the electron density defines the interatomic surfaces. The surface that the nitrogen of one amide bond forms with the neighbouring carbonyl carbon, denoted by -HN|, is at the centre bottom of the figure while the surface that the carbonyl carbon forms with the nitrogen of another amide bond, denoted by -(O=)C|, is on the left with the carbonyl oxygen atom at the top.
The serinyl group represented in Figure 1, is a space-filling object. It is represented in Figure 1 by the intersection of the isovalued envelope of the electron density that determines its van der Waals shape and size with the two interatomic surfaces that cut through the electron density between the carbonyl carbon and the amidic nitrogen atoms of the two amide bonds to give the fragment |NHCH(CH2OH)C(=O)|. The interatomic surfaces are defined in terms of a particular topological property of the electron density. This group can be linked to other such peptide units in a theoretical synthesis of a polypeptide or any portion of it. Each group has a zero net charge and a set of transferable properties that make additive contributions to the corresponding properties of the total system; to its energy, volume, electrostatic field and polarizability, for example. The theory of atoms in molecules enables one to take advantage of the single most important observation of chemistry, that of a functional group with a characteristic set of properties.
The topological basis of the theory and its relation to the quantum mechanics of an open system are outlined and illustrated in the document "Theory of Atoms in Molecules." Below is a list of references which provide reviews of the theory and illustrate its application.
References
- R. F. W. Bader, Atoms in Molecules - A Quantum Theory, Oxford University Press, Oxford, 1990.
- X. Fradera, M. A. Austen and R. F. W. Bader, The Lewis Model & Beyond, Journal of Physical Chem. A. 103, 304 - 314 (1999).
- R. F. W. Bader, A Bond Path - A Universal Indicator of Bonded Interactions, Journal of Physical Chemistry A, 102, 7314 - 7323 (1998).
- R. F. W. Bader, Why Are There Atoms In Chemistry?, Canadian Journal of Chemistry, 76, 973 - 988 (1998).
- R. F. W. Bader and M. A. Austen, Properties Of Atoms In Molecules: Atoms Under Pressure, Journal of Chemical Physics, 107, 4271 - 4285 (1997).
- R. F. W. Bader and J. A. Platts, Characterization Of An F-Centre In An Alkali Halide Crystal, Journal of Chemical Physics, 107, 8545 - 8553 (1997).
- I. Bytheway, R. J. Gillespie, T.-H. Tan g and R. F. W. Bader, Core Distortions and Geometries of the Difluorides and Dihydrides of Ca, Sr and Ba, Inorg. Chem. 34, 2407 (1995).
- R. F. W. Bader, P. L. A. Popelier and T. A. Keith, Theoretical Definition of a Functional Group and the Molecular Orbital Paradigm, Angewandte Chemie, Intl. Ed., Eng. 33, 620 (1994).
- R. F. W. Bader, Principle of Stationary Action and the Definition of a Proper Open System, Phys. Rev. B49, 13348 (1994).
- P. L. A. Popelier and R. F. W. Bader, The Effect of Twisting a Polypeptide on its Geometry and Electron Distribution, J. Phys. Chem. 98, 4473 (1994).
- P. F. Zou and R. F. W. Bader, Topological Definition of a Wigner-Seitz Cell and the Atomic Scattering Factor, Acta Cryst. A50, 714 (1994).
- R. F. W. Bader and T. A. Keith, Properties of Atoms In Molecules: Magnetic Susceptibilities, J. Chem. Phys. 99, 3683 (1993).
- T. A. Keith and R. F. W. Bader, Topological Analysis of Magnetically Induced Molecular Current Distributions, J. Chem. Phys. 99, 3669 (1993).
- R. F. W. Bader, K. M. Gough, K. E. Laidig and T. A. Keith, Properties of Atoms in Molecules: Additivity and Transferability of Group Polarizabilities, Mol. Phys. 75, 1167 (1992).
- C. Chang and R. F. W. Bader, Theoretical Construction of a Polypeptide, J. Phys. Chem. 96, 1654 (1992).
- R. F. W. Bader, R. J. Gillespie and P. J. MacDougall, Physical Basis for the VSEPR Model of Molecular Geometry, J. Am. Chem. Soc. 110, 7329 (1988).
- K. B. Wiberg, R. F. W. Bader and C. D. H. Lau, A Theoretical Analysis of Hydrocarbon Properties: II Additivity of Group Properties and the Origin of Strain Energy, J. Am. Chem. Soc. 109, 985 (1987).
"Theory
of Atoms in Molecules"
AIMAll Software
AIMPAC and Other AIM Software
Chemistry
Faculty
Department of
Chemistry
Last Updated: April 4, 2010