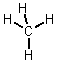
4 Subjects in Term 2
No more handouts for problems and tutorials - use the Web
http://www.chemistry.mcmaster.ca/courses/1aa3
Course notes: (I HOPE)
http://www.chemistry.mcmaster.ca/courses/1aa3/1aa3blue.htm
If you can't find them, go to
McMaster (www.mcmaster.ca), then follow the path to: i) Academic ii) Faculty of Science iii) Chemistry iv) Current Courses v) Chem 1AA3
Tutorials and labs start next week.
Bonds
Covalent 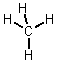
Ionic Li-Cl ![]()
Metallic Mn-Mn-Mn etc.
These bonds represent very strong forces 100 - 450 kJ/mol
By contrast, intermolecular forces are weak. Always < 100 kJ/mol. Typically 1-25 kJ/mol.
Thus, as one heats up a molecule, one overcomes weaker intermolecular forces first, only at high T, are strong intramolecular forces broken.
Table: Comparison of the Fahrenheit and Celsius temperature scales.
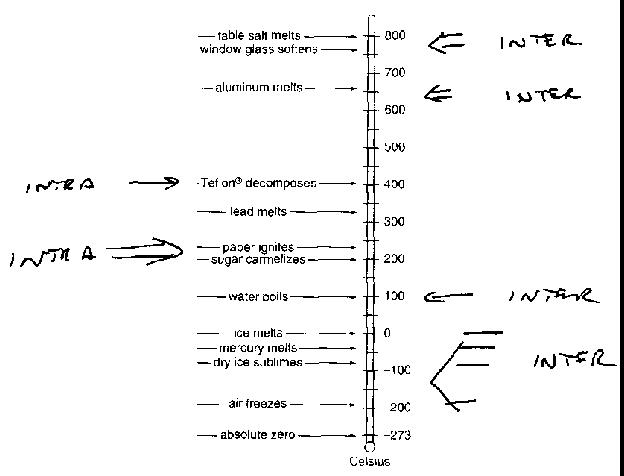
-essentially no intermolecular forces even in Li+Cl- vapour (there are strong ion-ion intramolecular forces, but weak LiCl - LiCl intermolecular forces)
fast molecular movement
rotate (ii) translate (iii) vibrate
- molecules close together
-rapid molecular movement
(i) rotate (ii) translate (iii) vibrate
- weak intermolecular forces
- ordered
(i) sometime rotate (ii) vibrate
- NO translation
- forces depend on crystal type
Ionic solid Very strong intermolecular forces
(may actually be considered intramolecular forces)
Molecular solid Very weak intermolecular forces
a) ion - ion
b) dipole - dipole
c) induced dipole - induced dipole
(London forces)
d) H bonding
F q2/r2 (charge / distance)
Ionic Solids A 3 D network of positive (+) and negative (-) charges
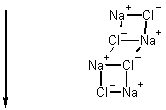 close together high mp
close together high mp
Ionic Liquids conduct electricity close together, very high bp
![]()
Ionic Gases separation of ion pairs, NaCl molecules are far apart from each other
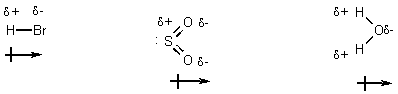
Polar Molecules
= (d+) (d-) / r
In a liquid
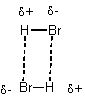
i)  (otherwise gases wouldn't
ever condense)
(otherwise gases wouldn't
ever condense)
ii) 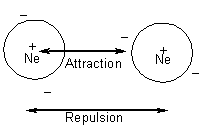 consider attraction
of electrons of 1 atom to nucleus of another. These forces are additive:
more points of contact, more attraction
consider attraction
of electrons of 1 atom to nucleus of another. These forces are additive:
more points of contact, more attraction
CH3CH2CH2CH2CH3 CH3CH(CH3)CH2CH3 CH3C(CH3)2CH3
b.p. 36 oC b.p. 28 oC b.p. 9o C
CH4 mp - 183oC C20H42 80 oC C100H202 135 oC
If molecules far apart, little interaction
if molecules close , attraction > repulsion
if molecules too close, repulsion >> attraction
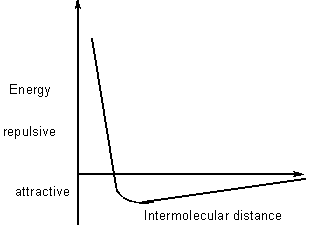
Factors affecting the magnitude of induced dipole interactions
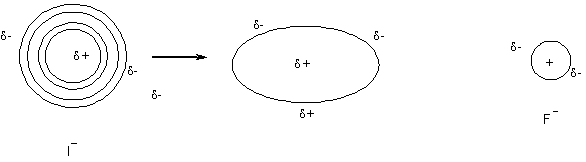
Polarizability
As ¯ Periodic table size polarizability
Why?
Outer electrons shielded from nucleus more affected by external stimuli
i.e., big, soft blobby I- is much more polarizable than hard small F-
- because H so small can approach close to atoms, so small migration easy
- stronger than London or Dipole forces (see Fig. 13.9 p. 605)
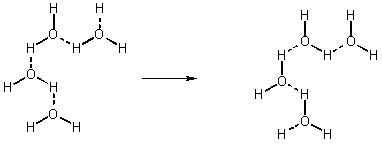

Magnitude of Forces London ~1 - 5 kJ/mol
Dipole ~1 - 10 kJ/mol
H-bond ~5 - 25 kJ/mol

NB: Polymers have glass transition
Demo: Latex rubber
Liquid N2 - Bunsen Burner
NaCl + Bunsen burner
Ice
CO2 - Balloon
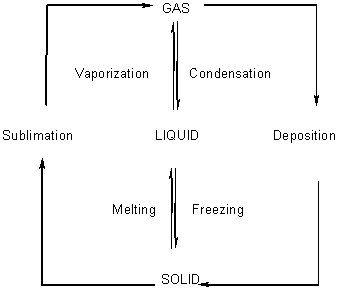
Freezing / Melting - Break up intermolecular forces.
Cost is proportional to the Strength of the intermolecular force
| m.p. C | b.p. C | |||
| H2 | 6.12 | -259 | ||
| CH4 | 6.94 | -183 | 10.4 | 164 |
| Hg | 2.3 | -40 | ||
| Ccl4 | 2.51 | -23 | 30 | 76.7 |
| EtOH | 5.01 | -115 | ||
| H2O | 6.01 | 0 | 40.7 | 100 |
| NaCl | 27.2 | 801 |
Evaporation / Condensation
Molar enthalpy of vaporization , ![]() Hv
Hv
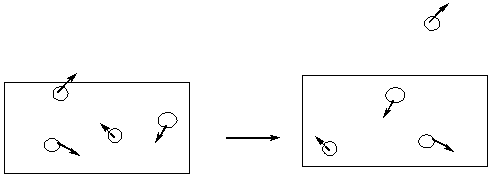
Escape velocity higher, vaporization ![]() Hv
lower
Hv
lower
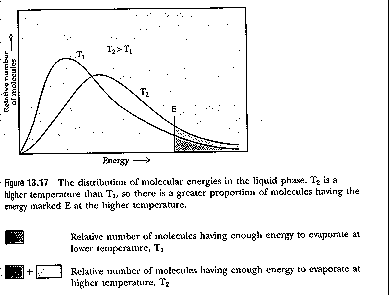
Molecules in liquids have energy distribution
Demo: pail test tubes and corks
Rubber duck
Tennis ball
What happens with a liquid in:
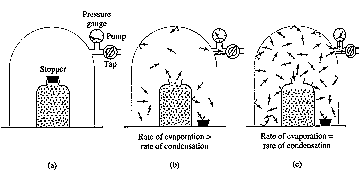
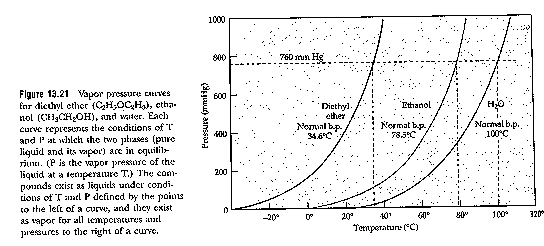
= as Temperature increases, so does the vapour pressure
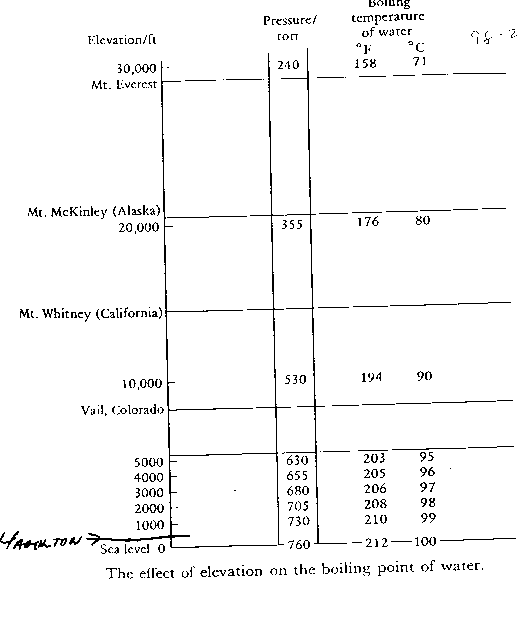
The effect of elevation on the boiling point of water
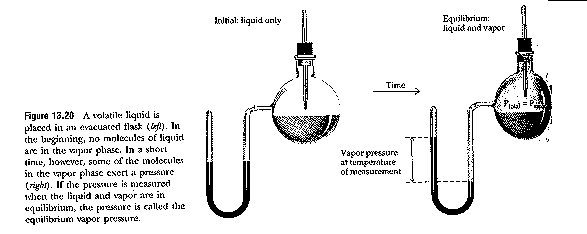
Solids do not have large vapour pressure
CO2(s) H2O @ 0 oC
very large Pv = 4.6 mm
(dry ice unusual) i.e. Frozen underwear sublimes
Some examples
1. bp of EtOH at 600 mm Hg from graph? (answer, look at graph p. 616, Pv ~ 74 oC)
2. If 1L H2O in 10L sealed flask at 60 C (Pv from graph of @ 60o = 180 mm Hg). How much H2O will evaporate?
PV = nRT
180 mmHg x 9L = n R 60 oC
(180mm Hg) / (760 mm Hg / atm) x 9L = n 0.082 L atm x 333 K / K mol
n = 0.078 moles of water will fill space in the 10 L vessel (1L of which is air). You can figure out how many grams that is.
Liq. N2
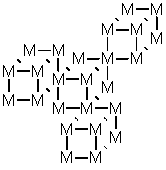
Network solid (covalent network crystal)
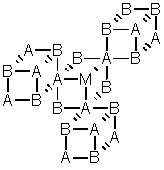
e.g.
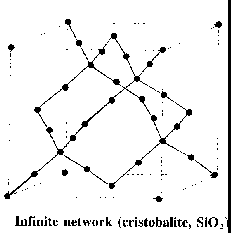
Molecular solid
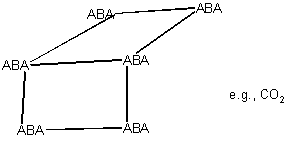
ABA = Molecule
e.g. CO2 A=O, B=C
Combination - Graphite
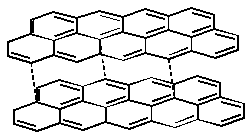
Diamond Eq 2 D network solid
In D molecular solid
3 common types
i) atoms at vertices, Simple Cubic
=8 apices x 1/8 atom
= 1 atom/unit cell
74% space consumed
ii) Body - centered cubic
(Fe, Na, Ba)
- 1 atom each apex
+ 1 in centre
= 1 atom centre
+ 8 x 1/8 (= 1)
Total 2 atoms /unit cells
68% space consumed
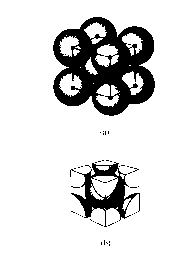
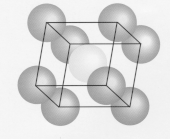
iii) Face centered Cubic
(Cu, Al, Ca, Ne, K)
1 atom each apex (o) and 1 atom at each face (o)
6 faces x ½ atom ( = 3 atoms)
+ 8 x ½ ( = 1 atom)
= 4 atoms/unit cell
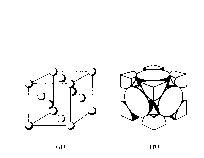
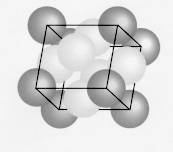
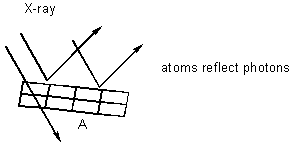
eg. Bragg Reflection
- can back calculate A. Using cell dimension, can calculate metal density
e.g. Density of Vanadium
- crystal body-centred cubic
- edge length 305 pm
- density = g/cm3
- volume of unit cell = Edge3 = 305 pm3
Mass (body centered cubic 2 atoms)
Density = Mass / volume
Mass = 2 atoms (50.94 g / mol) x 1 / 6.02 x 1023 atoms/mol = 1.692 x 10-22 g
Volume = (305 pm (1 cm / 1010 pm))3
Density = 5.96 g / cm3
If you know density and unit cell can calculate Avagadro's number (see tutorial)
What about non-crystalline materials, amorphous materials don't have a narrow melting point
e.g. glass