| McMaster University - Chem2O06 Lab Manual | 1997/98 |
Experiment 2. Distillation - Introduction
Distillation is the most common method used to separate and purify liquids. The process consists of heating a liquid to its boiling point, conducting the vapours to a cooling device where they are allowed to condense, and collecting the condensate.
General Principles
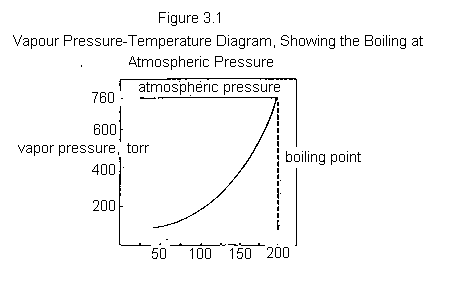
If a liquid is kept in a sealed container, some molecules escape from the liquid's surface into the space above it. When equilibrium is established, the number of molecules escaping the liquid equals the number of molecules in the vapour that strike the liquid surface and stick. The molecules in the vapour also strike the walls of the container and exert a pressure, defined as the vapour pressure of the liquid. If the temperature of the liquid is raised, a greater number of molecules escape to the vapour phase until equilibrium is once again established; the vapour pressure of the liquid increases with increasing temperature. Figure 3.1 shows a typical vapour pressure-temperature curve.

l. The Boiling Point
The boiling point of a liquid is that temperature at which the vapour pressure of the liquid is equal to the pressure of its surroundings. If the flask that contains the liquid is open to the atmosphere, the boiling point will be the temperature at which the vapour pressure of the liquid is equal to atmospheric pressure. The vapour pressure of a pure liquid rises steadily as the temperature is increased, until the boiling point is reached (Figure 3.1).
A thermometer placed in the vapour of a boiling pure liquid will record its boiling point. If this is done in a distillation apparatus (see Figure 3-5), the temperature will remain constant throughout the distillation. This is because at the boiling point, vapour and liquid are in equilibrium, and, if the composition of the vapour and liquid remains constant through the process, the temperature will also remain constant. The boiling point (at a given pressure) is a characteristic property of a pure liquid, just as the melting point is a characteristic property of a pure crystalline solid. However, in contrast to melting points, the pressure must always be recorded when determining a boiling point.
2. A Mixture of Ideal Liquids
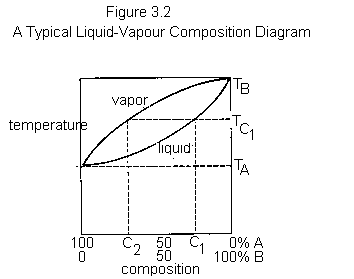
If a mixture of two miscible liquids with different boiling points is heated to boiling, the vapour will not have the same composition as the liquid; it will be richer in the more volatile component. Consider Figure 3.2, which depicts the behaviour of mixtures of A and B, two miscible volatile liquids with boiling points TA and TB, respectively. The lower of the two solid curves represents the boiling points of mixtures of A and B. The upper curve represents the composition of the vapour which is in equilibrium with the liquid as its boiling point. At 100% A or 100% B the curves meet, because boiling pure A (at TA) can have only pure A vapour in equilibrium with it; the same applies to pure B (at TB).
If a mixture of A and B with composition C1 is heated, it will boil at TC1. Reading horizontally on the graph, one sees that the vapour at TC1 will have the composition given by C2. This means that if mixture C1 were placed in a distillation apparatus and heated to its boiling point, the vapour (and therefore the first drop of liquid to be condensed) would have the composition C2; it would be much richer in A, the more volatile of the two components, than was the original liquid.
As the distillation proceeded, A would be selectively removed from the liquid. The composition of the liquid would change gradually from C1 to 100% B. The boiling point of the liquid would gradually rise from TC1 to TB; at the same time, the composition of the distillate would gradually change from C2 (rich in A) to 100% B. Thus, in a simple distillation of a mixture, the first material to distill (sometimes called the first cuts or first fractions) will be rich in the most volatile or lowerboiling components and the last material (last cuts or fractions) will be rich in the less volatile or higherboiling components.
This behaviour is true for "ideal liquids" with no intermolecular interactions, and is approximated by many organic mixtures.
3. Liquids that Form Azeotropes
Some liquid mixtures, instead of forming ideal solutions with liquidvapour composition diagrams typified by Figure 3.2, form either minimum or maximum boiling mixtures. Typical composition diagrams for such mixtures are shown in Figure 3.3. Any mixture with a composition exactly equal to that of CMIN or CMAX will distill at a single constant temperature, just as if it were a pure liquid. Ethanol and water form a constant boiling mixture (95.6% ethanol, 4.4% water) with a minimum boiling point of 78.2o C (lower than that of pure ethanol, 78.3o C, or pure water, 100o C). Formic acid and water also form a constant boiling mixture (22.5% formic acid, 77.5% water) with a maximum boiling point of 107.1 o C (higher than that of formic acid, 100.8
Mixtures with a composition to the left of CMIN or CMAX, in Figure 3.3, can be separated into pure A and the constant boiling mixture, but pure B cannot be obtained from such a mixture by distillation. Conversely, mixtures with a composition to the right of CMIN or CMAX, in Figure 3.3, can be separated into pure B and the constant boiling mixture, but can never furnish pure A by distillation.
Fractional Distillation
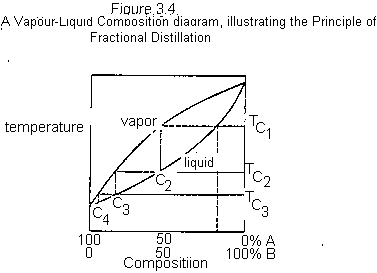
It was noted from Figure 3.2 that even the first drop of distillate to be obtained when an AB mixture with composition C1 was distilled did not give pure A, but rather a mixture C2, containing mainly A but some B. If these first fractions were to be combined and redistilled, the first vapour to be condensed would be even richer in A. Repetition of this process (vaporization, condensation, and revaporization) could eventually lead to isolation of pure A from the AB mixture. Similar redistillation of the higherboiling fractions could lead to isolation of pure B in the final fractions. Clearly this repeated redistillation is a laborious process.
The fractionating column (Figure 3.6) is a device for increasing the efficiency of this redistillation process. It consists of a vertical column packed with some inert material, such as glass beads or glass helices, or provided with some other device (indentations) for increasing the surface upon which the vapour may condense. As the hot vapours rise through the column, they condense and flow back down the column. The condensate, as it hits the lower, hotter portions of the column, is revaporized, and the more volatile components proceed up the column once again. If the column is efficient, this process is repeated many times in the column, and the distillate will consists of the lowestboiling components of the mixture in nearly pure form. Figure 3.4 illustrates the process graphically. The original AB mixture with composition C1 boils at temperature TC1 and the vapours enter the column at that temperature. If they condense in the column, the condensate will have the composition C2. This vaporizes near the bottom of the column at temperature TC2, producing vapours with composition C3. These may condense further up the column at TC3; vaporization now gives vapour with composition C4, etc. If the column contains enough surface area for many successive vaporizations and condensations, the distillate that comes over will be
nearly pure A with a boiling point close to that of pure A. This will continue until all of A is removed, after which B will start distilling, and the temperature will rise rather abruptly to the boiling point of B. In practice, distilling columns are not 100% efficient, but columns have been designed which can separate liquids that boil as little as 2 apart.
17sep97; wjl 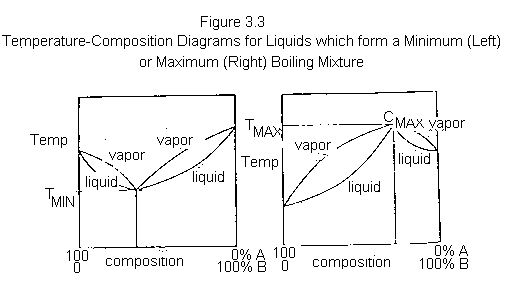
Go to:
Instructions for Printing this Document
Experiment 3 - Main Page
Procedures
Chem2O06 Home Page.