| McMaster University - Chem2O06 Lab Manual | 1997/98 |
Experiment 3, Part B: Procedures.
Separation of a Binary Mixture by Simple Distillation at Atmospheric Pressure Using a Fractionating Column
PROCEDURE
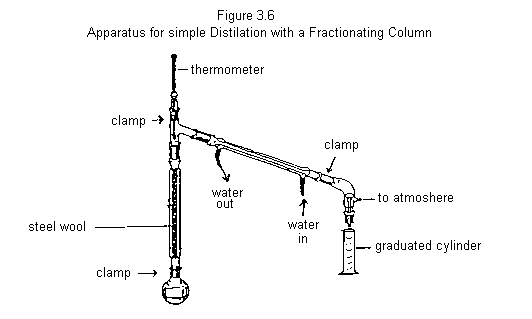
Assemble the apparatus shown in Figure 3.6; again use your graduated cylinder to collect the distillate. (Only replace the steel wool in the condenser if it is severely rusted. Check with your T.A. if in doubt.)

Place a mixture containing 25 mL of each of cyclohexane and p-xylene in a 100 mL round-bottomed flask (dont forget some boiling chips!), connect it to the fractionating column (Fig. 3.6) and proceed as described above. [NOTE that you will need to start the distillation under fairly high heat, otherwise you will not finish on time.] Again, record the temperature every 2 mL as the distillation proceeds until 45 mL of distillate are collected. Be sure to increase the heating rate near the midpoint of the distillation otherwise the head temperature will drop. (Why?)
Recording of Results
Construct a table in your notebook like that given below, to record the temperature at the distillation "head" as a function of volume distilled. You will record your data in both your and your partner's notebook simultaneously. He/she will do the same.
| Volume distilled (mL) | 2 | 4 | 6 | 8 | 10 | 12 | 14 | 16 | 18 | Temperature without column | Temperature with column |
As the table is being filled out, plot the boiling point vs. volume distilled for the distillation of the cyclohexane/p-xylene mixture with and without the fractionating column. Both sets of data will be plotted on the same graph, using different symbols. Label the two curves.
Note that no azeotrope is formed in this experiment. Also note that you are plotting temperature vs. volume not temperature vs. composition as is shown in Fig. 3.2. These plots are not expected to be directly related.
Questions: 1. On the basis of your results, which procedure was more efficient at separating the mixture into its components? 2. From your results what generalization can you formulate about the purity of a liquid as judged by its boiling point? 3. Define the term boiling point. What effect would a reduction in the atmospheric pressure have on the boiling point of a liquid? 4. Why would it be dangerous to heat an organic compound in a distilling apparatus that was closed tightly at every joint and having no vent or opening to the atmosphere or to a vacuum pump? Begin this Experiment after you have begun the distillation in Experiment 3. Work in PAIRS, using the same partner as for the distillation.
In the following procedure, it is important that the column be mounted as close to vertical as possible. Also, all surfaces and interfaces must be as flat and even as possible.
In this experiment you will use a Pasteur pipette to prepare a chromatographic column and separate 5 mg samples of fluorene and fluorenone. You will also use thin layer chromatography to analyze the 1 mL fractions collected during the elution of the compounds to determine the degree of separation and the purity of the compounds.
Procedure
Your TA will use the Bunsen burner set up in the hood to close the tip of a `long' Pasteur pipette and make a small bend in the closed tip. Place the pipette in the split rubber stopper and clamp the assembly. Place a small wad of glass wool into the bottom of the pipette, pour in some sand (0.3 cm), fill it half-full with pure Ligroin*, pack the `column' with alumina (5 cm), and pour sand (0.3 cm) on top of the alumina. Take 1 mL of the fluorene/fluorenone solution provided in 1 mL of pet-ether. Break off the tip of the `column' (hold the pipette near the bend when breaking) and when the Ligroin just disappears through the sand add the solution of fluorene/fluorenone to the top of the column dropwise until the sample has been loaded on the column.
When the final volume of fluorene/fluorenone solution disappears through the sand at the top, add elution solvent (4:1 Ligroin/ether) to the top of the column dropwise (about 10 drops), then fill the column to the top. (Do not let the solvent level drop below the sand. Channels will develop in the alumina resulting in a poor separation of the compounds.) Collect at least five (5) one mL fractions in vials. Prepare some capillary applicators, spot the fractions on alumina tlc strips along with fluorene and fluorenone and develop the strips in a bottle containing a 5:1 mixture of pet-ether/ether. Visualize the chromatograms with the ultraviolet lamp. Combine the pure fractions of the compounds and evaporate the solvent in the hood. Place the solid residue in the appropriate "student collection bottle".
[If your fluorenone has not appeared by fraction 5 (light yellow colour) collect 2 - 3 more 1 mL fractions.]
1. Draw out the structures of fluorene and fluorenone.
2. What would be the result of the following errors in TLC technique?
(a) Too much sample applied?
(b) Polarity of solvent too low?
(c) Solvent pool in developing jar too deep?
(d) Allowing the solvent front to proceed to the top of the plate?
| Go to: | Instructions for Printing this Document Experiment 2 - Main Page Introduction Part A Procedures Chem2O06 Home Page. |
16sep97; wjl