| MidTerm Test #1 | ANSWERS | November 22, 1996 |
Please see your Tutorial Leader before January 15, 1997 if there is an addition error in your paper, or if you believe that you were robbed of a significant (i.e. >5) marks.
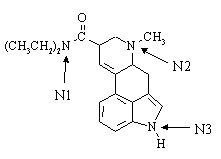
1. Lysergic acid N,N-diethylamide ("LSD") has the general structure shown below:

(a) The condensed formula of LSD is C20H25N3O.
(b) The hybridization of the three nitrogen atoms are N1: sp2; N2: sp3; N3: sp2.
(c) The most basic nitrogen atom in the molecule is N2.
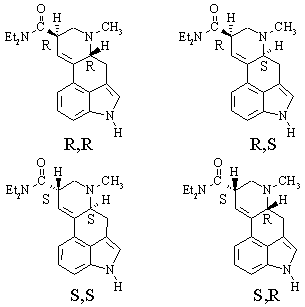
(d) How many stereocenters does LSD possess? - two. How many configurational isomers are there for a molecule of this general structure? - four. How many of them are chiral ? - all of them
(e) Draw the structure of one of the configurational isomers of LSD. Label the stereocenters in your structure with its absolute configuration, determined using the Cahn-Ingold-Prelog rules.
(f) LSD reacts with one equivalent of bromine in carbon tetrachloride. In the structure you have drawn for part (e), identify the site in the molecule which is expected to be most reactive towards Br2. Draw the structure of the major product(s) expected from the reaction of the particular stereoisomer you have drawn in (e) with this reagent:
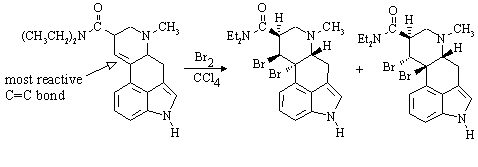
All the other double bonds are aromatic!
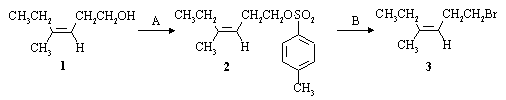
A laboratory synthesis of the sex pheromone of the codling moth involved the following reactions:
See Ege, Problem 8.46
(a) Name the alkene (1) used as starting material in the synthesis, including its stereochemistry:
Z-4-methyl-3-hexen-1-ol
(b) Supply the reagents and solvents necessary to carry out the two steps of the transformation shown above.
A: 4-toluenesulfonyl chloride (TsCl) in pyridine.
B: NaBr in acetone, methanol, ethanol, acetone, or THF.
(c) Alcohols can also be converted to alkyl halides by reaction with the corresponding hydrohalic acid. For example,
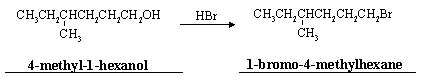
(i) Write the names of these two compounds (see above).
(ii) Write a complete mechanism for this reaction.
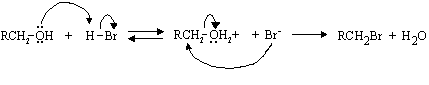
(iii) Why can't this procedure be used for the direct conversion of 1 to 3 in the pheromone synthesis on the previous page?
Because the C=C bond would react.
Draw the products you would expect, and the mechanisms by which they are formed:
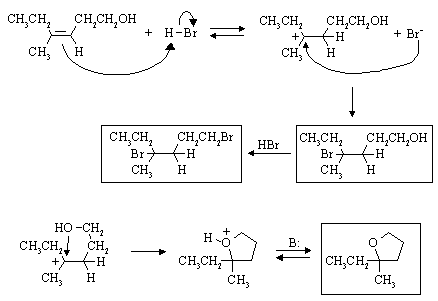
See: Ege, Problem 7.40
This is E2 elimination. In cis-1-bromo-2-phenylcyclohexane, the H and the leaving group
can assume the anti-periplanar arrangement which is required for fast elimination. In the
trans-compound, this is not possible. (Syn-) elimination to give the same alkene is much slower than from
the cis-isomer. However, it is favoured over anti-elimination on the other side because
the nonconjugated alkene that would result (3-phenylcyclohexene)
is much less stable than 1-phenylcyclohexene.
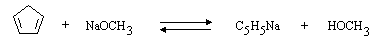
(a) Given that for methanol, pKa = 15.5, the pKa of cyclopentadiene is (circle the correct answer; R = 8.3 J mol-1 K-1):
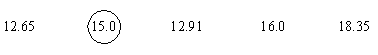
Rough work:
Keq = KaCP / KaMeOH (CP = cyclopentadiene; MeOH = methanol)![]() Go = -RTln Keq = -2.303RTlog Keq
Go = -RTln Keq = -2.303RTlog Keq
log Keq = (-2850)/[(-2.303)(8.3)(298)] = 0.5
log Keq = log KaCP - log KaMeOH
= pKaMeOH - pKaCP
pKaCP = pKaMeOH - log Keq
= 15.0
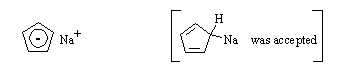
(b) In tetrahydrofuran (C4H8O) solution, which is present in higher concentration, C5H6 or C5H5Na? C5H5Na.
(c) In methanol solution, which is present in higher concentration, C5H6 or C5H5Na? C5H6.
(d) Draw the structure of C5H5Na:
(e) Cyclopentadiene is unusually acidic for a hydrocarbon. Draw all the resonance structures for its conjugate base. Why is C5H6 so acidic?
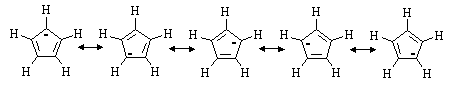
The five carbons in the cyclopentadienyl anion are all sp2 hybridized.
Thus, the ![]() -system consists of a cyclic array of p-orbitals containing
six electrons - the molecule is AROMATIC!
-system consists of a cyclic array of p-orbitals containing
six electrons - the molecule is AROMATIC!

| Go to: |
Instructions for Printing this Document Chem2O6 Problem Sets & Answers Chem2O6 Home Page. |
06dec96; wjl