Chem2O6
- 1997/98| MidTerm Test #1 | ANSWERS | November 21, 1997 |
Please see your Tutorial Leader before January 15, 1998 if there is an addition error in your paper, or if you believe that you were robbed of a significant (i.e. >5) number of marks.
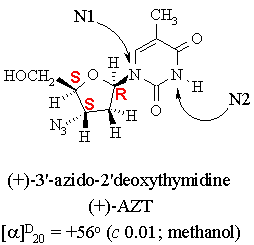
1. The structure of AZT, a drug used with some success in the treatment of AIDS, is shown below.

This question was adapted from Ege, p289, #7.49.
(a) The condensed formula of AZT is C10H13N5O4.
(b) The hybridization of the two labelled nitrogen atoms are N1: sp2; N2: sp2.
(c) At some point, the preparation of this compound must have involved a SN2 reaction with azide ion. Draw the major resonance contributors for the azide ion:
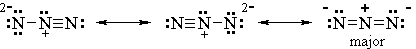
(d) Draw the structure of (-)-AZT, the enantiomer of (+)-AZT.
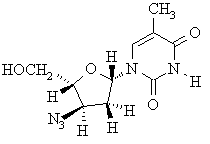
(e) Indicate the configuration of all of the stereocenters in (+)-AZT on the structure given above.
(f) A partially resolved mixture of (+)- and (-)-AZT exhibits an optical rotation of -14o. The diastereomeric composition of this mixture is 37.5 % (+)- and 62.5 % (-)-AZT. The enantiomeric excess of the (-)-enantiomer is 25% .
(g) The total number of stereoisomers possible for a compound having the same connectivity as AZT is 23 = 8 .
(h) The following reaction is used in preparing AZT:
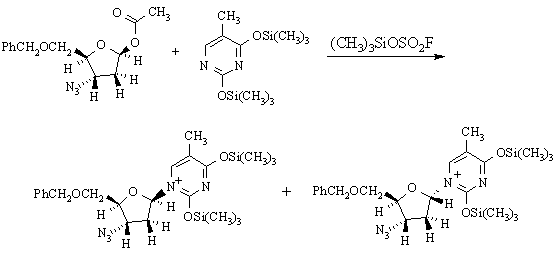
The two products are: (A) enantiomers; (B) diastereomers; (C) constitutional isomers.
What reaction mechanism is implied by the products obtained in this reaction? Why?
SN1 substituion, because the stereochemistry at the reaction center has randomized.
What does this tell you about the properties of (CH3)3SiOSO2F as a reaction solvent? It's polar & ionizing.
Draw a mechanism for the reaction which illustrates how the two products are formed. What (other than the solvent) makes this mechanism favoured over other possible mechanism(s)?
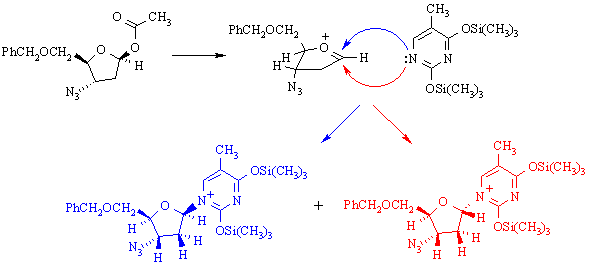
The secondary carbenium ion formed in the first step is resonance-stabilized:
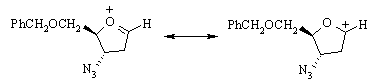
2. Both the cis and the trans isomers of 3-methoxycyclohexanol are found as components of a group of antibiotics called macrolides. When one of the isomers is treated with base followed by iodomethane, the product is an optically inactive 1,3-dimethoxycyclohexane. Reaction of the other diastereomer under the same conditions gives a racemic mixture of optically active 1,3-dimethoxycyclohexanes. (The "methoxy" group is -OCH3)
(a) Draw a good 3-dimensional representation of a stereoisomer of 3-methoxycyclohexanol that would give optically inactive 1,3-dimethoxycyclohexane when treated with base and methyl iodide. Indicate whether it is chiral or achiral.
See Ege, Problem 6.36 - part a
(b) Suggest and draw the structures of a base and solvent(s) for the reaction of part (a), and write the complete mechanism for the conversion of 3-methoxycyclohexanol to 1,3-dimethoxycyclohexane.
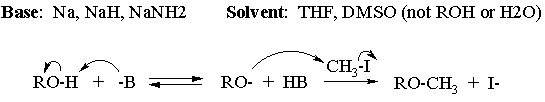
(c) Do you expect to have problems from competing elimination? NO Explain why or why not. Elimination would require loss of OH- or CH3O- as leaving groups - they're both poor leaving groups.
(d) Draw a good 3-dimensional representation of a stereoisomer of 3-methoxycyclohexanol that would give optically active 1,3-dimethoxycyclohexane when treated with base and methyl iodide. Indicate whether it is chiral or achiral.
See Ege, Problem 6.36 - part b
(d) Draw the possible conformers of cis- and trans-1,3-dimethoxycyclohexane. For each of the two compounds, identify the most stable conformer.
See Ege, Problem 6.36 - part c
3. (a) One good way of synthesizing alkenyl halides is by a two step route involving addition of X2 followed by elimination of HX. For example,
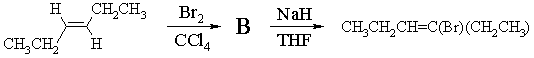
(i) Draw a 3-dimensional structure of B, the product of bromination of E-3-hexene, and illustrate the mechanism by which it is formed.
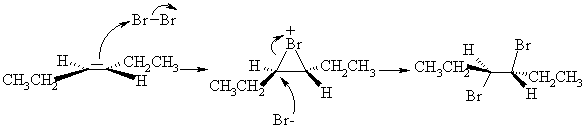
(ii) Draw the structure of the other regioisomer which could, in principle, be formed in the elimination reaction. Give two reasons why the product shown above is the one formed in highest yield.
The product above is major because (1) it is the more highly substituted, therefore more stable, and therefore formed faster; and (2) its formation involves abstraction of the more acidic H.
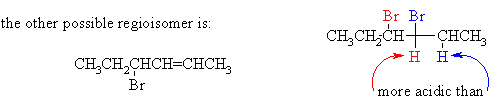
(iii) Draw the structure of the isomer of CH2CH2CH=C(Br)(CH2CH3) you expect to be formed in this reaction, clearly showing its stereochemistry. With the help of a Newman projection, illustrate the mechanism by which it is formed from reaction of B with NaH.
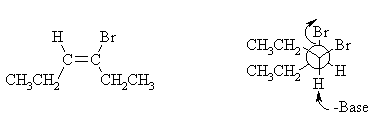
(b) Menthyl chloride and neomenthyl chloride differ from one another only in the stereochemistry at the carbon atom to which the chlorine atom is attached. The two alkyl groups in both compounds are trans to one another. Treatment of the two compounds with sodium ethoxide in ethanol leads to the following products....
See Ege, Problem 7.41
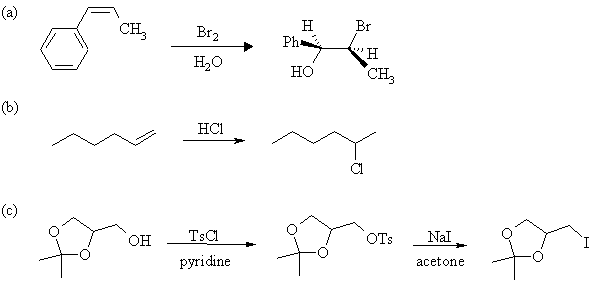
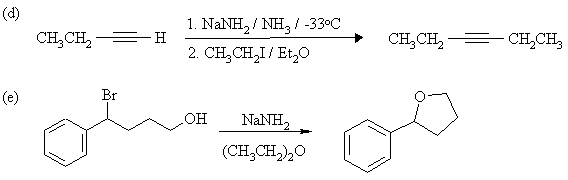
4. Complete the following reactions, showing stereochemistry where appropriate. If more than one product is formed, indicate which one(s) are major. Mechanisms are not necessary.
5. The following sequence of reactions was used for the conversion of 1-phenylethanol to 1-cyano-2-phenylethane:
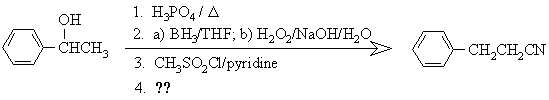
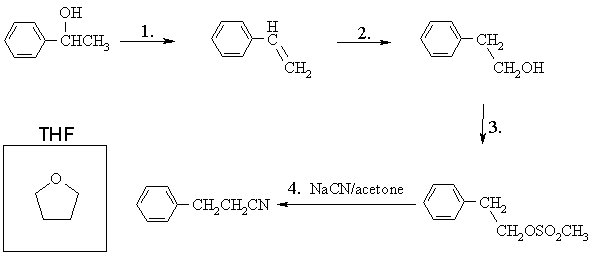
(a) In the spaces below, draw structures of the products of the first three transformations, the reagents one should use for the fourth transformation, and in the box provided, the structure of THF (tetrahydrofuran, C4H8O)
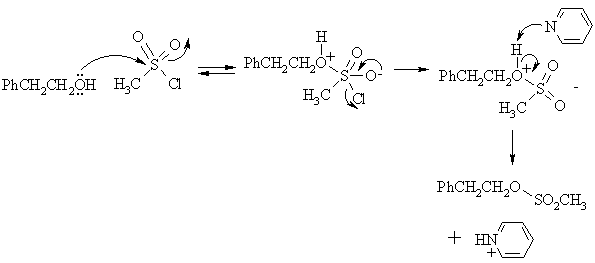
(b) Write the mechanism for Step 3 of the above sequence.
6. Name the following compounds according to IUPAC nomenclature, specifying stereochemistry (where shown) using Cahn-Ingold-Prelog nomenclature:
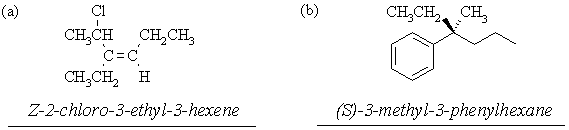
7. Suggest the cleanest possible synthesis of the following compound, from the compounds given and any other reagents you require. More than one step may be necessary. Identify any minor products expected in your synthesis.
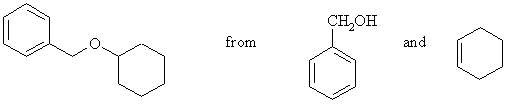
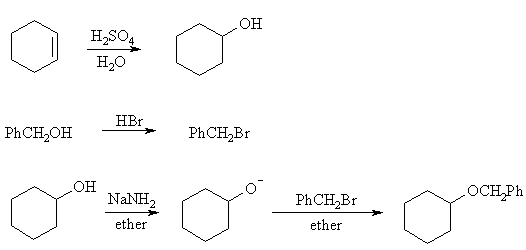
Ethers are synthesized by the SN2 reaction of an alkoxide and an alkyl halide. An ether R-O-R' can thus be made from RO- + R'X or RX + R'O-. Applied to this problem, the choices are then PhCH2X + C6H11O- or PhCH2O- + C6H11X . The first is better:
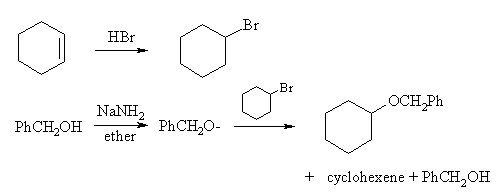
The second consists of one fewer step, but has problems in the last step due to competing elimination:
| Go to: | Instructions for Printing this Document Chem2O6 Problem Sets & Answers Chem2O6 Home Page. |
27nov97; wjl