| McMaster University - Chem2OB3 Lab Manual |
Experiment 1b. Isolation of Essential Oils by Preparative Gas Chromatography
In this experiment, we isolate (+)-carvone from caraway seed oil or (-)-carvone from spearmint oil, using preparative gas chromatography. (+)- and (-)-carvone are enantiomers, and their odors are distinctly different from each other. This is to be expected, since the human body distinguishes odor by the interaction of molecules with odor receptors in the nose, which are chiral. The phenomenon in which a chiral receptor interacts differently with each of the enantiomers of a chiral compound is called chiral recognition. Another example of chiral recognition can be found in the toxicity of the two carvone isomers; the toxicity of (+)-carvone in rats is 400 times greater than that of (-)-carvone.

With the exception of their optical rotations, the physical properties of enantiomers are identical. Thus, we predict that the boiling points, refractive indices, gas chromatographic retention times, infrared and nuclear magnetic resonance spectra of (+)- and (-)-carvone should all be identical. The only difference in properties that one should observe are the odors and the signs of optical rotation in a polarimeter.
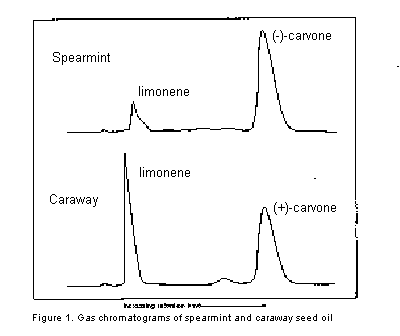
Caraway seed oil contains mainly limonene and (+)-carvone. The (+)-carvone (bp 230 oC) can easily be separated from the lower-boiling limonene (bp 177 oC) by gas chromatography, as illustrated in the GC-traces shown below in Figure 1. Using gas collection tubes, the two major components of the natural oil can be collected separately as they elute from the column. Spearmint oil contains mainly (-)-carvone, with a smaller amount of limonene and the isomeric terpenes, a- and b-phellandrene. The gas chromatogram of this oil is also shown in Figure 1. In this case, while it is easy to collect a pure sample of the carvone, limonene cannot be collected in pure form because it will be contaminated by the other terpenes, which have similar boiling points.
Your instructor will either assign you spearnint or caraway oil, or have you choose one of the oils. You will also be given instructions on which optional procedures, if any, you are to perform. Your instructor may have you isolate both the carvone and limonene components or only carvone. Avoid contact with (+)-carvone, as it is very toxic.
Gas Chromatography
In this experiment, you will carry out all aspects of the gas chromatographic separation yourself. The most critical part of the operation is the injection procedure - it is important that once you have pierced the injector with the syringe containing the mixture to be separated, you inject the mixture rapidly. Your T.A. will demonstrate the correct procedure for you; practice on your own several times with the small containers provided in the GC room, using the ethanol solutions provided, until you are confident that you can do it properly.
Inject 50-
mL of caraway-seed or spearmint oil onto the gas chromatography column. Just before a component of the oil (limonene or carvone) elutes from the column, install a gas collection tube at the exit port. To determine when to connect the gas collection tube, refer to the chromatograms on the wall of the GC room. These chromatograms have been run on the same instrument you are using under the same conditions. Ideally, you should connect the gas collection tube just before the limonene or carvone elutes from the column and remove the tube as soon as all the component has been collected, but before any other compound begins to elute from the column. This can be accomplished most easily by watching the recorder as your sample passes through the column. The collection tube is connected (if possible) just before a peak is produced, or as soon as a deflection in the pen is observed. When the pen has returned to the base line, the gas collection tube is removed.This procedure is relatively easy for collecting the carvone component of both oils and for collecting limonene in caraway-seed oil. Because of the presence of several terpenes in spearmint oil, it is somewhat more difficult to isolate a pure sample of limonene from spearmint oil (see chromatogram in figure). In this case, you must try to collect only the limonene component and not any other compounds, such as the terpene which produces a shoulder on the limonene peak in the chromatogram for spearmint oil.
After collecting the samples, insert the ground joint of the collection tube into a 0.1 mL conical vial, using an O-ring and cap to fasten the two pieces together securely. Insert the top of the collection tube through the hole in the rubber septum cap and place this assembly into a centrifuge tube. Put cotton on the bottom of the tube to prevent breakage. Balance the centrifuge by placing a tube of equal weight on the opposite side. During centrifugation, the sample is forced into the bottom of the conical vial.
Disassemble the apparatus, cap the vial, and perform the analyses described below.
ANALYSIS OF THE CARVONES
The samples obtained by gas chromatography and centrifugation should be analyzed by the methods below. Compare your results with those obtained by someone who used a different oil. In addition, measure the optical rotations of the commercial samples of (+)-carvone and (-)-carvone.
Gas Chromatography. Determine the retention times of the components. Calculate the percentage composition of the carvone sample by the triangulation method. In this method, a GC peak is approximated as a triangle, the area of which is proportional to the amount of material detected. The area of the peak is approximately equal to ½ X (peak height) X (peak width at half-height).
Infrared Spectroscopy. Obtain the infrared spectrum of the (-)-carvone sample from spearmint or of the (+)-carvone sample from caraway. Also obtain the infrared spectrum of the (+)-limonene, which is found in both oils. Determine both spectra on neat samples. If absolutely necessary, you may add one drop of nujol to the sample and mix it thoroughly before running the spectrum. The spectrum will contain extra C-H absorptions at around 3000 cm-1, however, as nujol (a.k.a. mineral oil) is a mixture of long chain aliphatic hydrocarbons.
Polarimetry. With the help of the T.A., measure the optical rotation of the (+)-carvone and (-)-carvone samples provided. Calculate the specific rotation of each compound (B&F, pp. 140-142). The concentration c will equal the density of the substances anaiyzed at 20 oC. The values are 0.9608 g/mL for (+)-carvone and 0.9593 g/mL for (-)-carvone. The literature values for the specific rotations are as follows:
[
aD20] = +61.7' for (+)-carvoneThese values are not identical because trace amounts of impurities are present.
Go to: Carvone NMR Spectra and Questions
| Go to: | Instructions for Printing this Document Chem2OB3 Home Page. |
23dec99; jp