The carbon-oxygen bond in ketones such as benzophenone is polarized, and the unsymmetrical distribution of charge, together with the unsaturation represented by the ![]() -bond, is a major consideration in rationalising the thermal (ground state) reactions of such compounds. Many reactions of ketones can be represented in terms of initial attack by a nucleophile at the partially positively charged carbon atom of the carbonyl group. Nucleophilic attack produces an oxygen-centred anion, which often goes on to abstract a proton in yielding the observed product. The proton source may be present in the reaction medium, or it may be made available in the work-up procedure.
-bond, is a major consideration in rationalising the thermal (ground state) reactions of such compounds. Many reactions of ketones can be represented in terms of initial attack by a nucleophile at the partially positively charged carbon atom of the carbonyl group. Nucleophilic attack produces an oxygen-centred anion, which often goes on to abstract a proton in yielding the observed product. The proton source may be present in the reaction medium, or it may be made available in the work-up procedure.
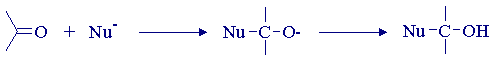
Reduction of ketones with metal hydride reagents is an example of such a reaction, and reduction of benzophenone using sodium borohydride leads to benzhydrol (diphenylmethanol):
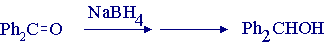
Ultraviolet irradiation of benzophenone leads to photochemical reaction originating in an electronically excited state, an (n,![]() *) triplet state, rather than in the ground state. This excited state does not have such a large polarisation of electrons in the C=O bond as in the ground state. Instead, its chief electronic feature is the odd-electron character of the oxygen atom, and the photochemistry of benzophenone can be rationalised on the basis of processes derived from an oxygen-centred radical-like species. One of the most ubiquitous reactions of free radicals is hydrogen atom abstraction; this is also the case for the excited triplet state of aromatic ketones.
It follows that effective reducing agents for the photochemical reduction of benzophenone are hydrogen atom donors rather than hydride ion donors. Primary and secondary alcohols are examples of good hydrogen atom donors, since the radical obtained by hydrogen atom abstraction from the carbon atom carrying the hydroxyl group is stabilised relative to a simple alkyl radical:
*) triplet state, rather than in the ground state. This excited state does not have such a large polarisation of electrons in the C=O bond as in the ground state. Instead, its chief electronic feature is the odd-electron character of the oxygen atom, and the photochemistry of benzophenone can be rationalised on the basis of processes derived from an oxygen-centred radical-like species. One of the most ubiquitous reactions of free radicals is hydrogen atom abstraction; this is also the case for the excited triplet state of aromatic ketones.
It follows that effective reducing agents for the photochemical reduction of benzophenone are hydrogen atom donors rather than hydride ion donors. Primary and secondary alcohols are examples of good hydrogen atom donors, since the radical obtained by hydrogen atom abstraction from the carbon atom carrying the hydroxyl group is stabilised relative to a simple alkyl radical:
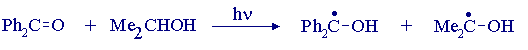
The purpose of this experiment is to demonstrate the effectiveness of using alcohols and UV light to carry out carbonyl reductions. We will compare the results with those of a sodium borohydride reduction to determine the differences between the two procedures.
PROCEDURES
(i) Photochemical reductionDissolve benzophenone (4.0 g) in 2-propanol (35 mL) by swirling in a 125 mL Erlenmeyer flask and warming on the steam bath. Add one drop of glacial acetic acid, to neutralise alkaline impurities, and fill a Pyrex test tube with the solution to within 2 cm of the top. Cork the tube loosely, and label it with your name using a grease pencil or magic marker. Give your tube to your demonstrator, who will irradiate it along with several others using a medium pressure mercury lamp for 3-4 hours.
Once you've recovered your solution, filter the precipitated product from the solution using a Buchner funnel, wash with cold ethanol and air-dry. Recrystallize the product from acetone, and record the yield, melting point and IR spectrum.*
(ii) Sodium borohydride reduction
In a 250 mL round-bottom flask, prepare a solution of benzophenone (1.0 g) in aqueous ethanol (8 mL ethanol + 2 mL water). Add sodium borohydride (0.25 g) and swirl the flask to assist solution. After about 30-40 minutes add ice-water (100 mL) and extract the product into ether (3 X 25 mL). Wash the combined ether extracts with aqueous 2M hydrochloric acid (40 mL) and then with water (40 mL). Dry the solution with anhydrous sodium sulfate, filter, and isolate the crude product using the rotary evaporator or a distillation apparatus. Recrystallize the product from a minimum amount of petroleum ether. Record the yield, melting point, and IR spectrum of the product.
QUESTIONS
1. Comment on the results of this experiment - are the products of the two reduction procedures the same? Identify the product(s), using the melting points and IR spectra to guide your assignment(s).2. Write mechanisms to account for product formation in the two experiments.
* IR spectra of liquids should be recorded as a neat film. Those of solids should be recorded in CCl4 solution or as a KBr pellet if the material is insoluble in CCl4. Nujol mulls are not used by organic chemists. All spectra are to be calibrated with the 1601.9 cm-1 polystyrene absorption.
| Go to: |
Instructions for Printing this Document Chem3D03 Lab Manual. |
30dec96; wjl