An Introduction to the Electronic Structure of Atoms and Molecules
Professor of Chemistry / McMaster University / Hamilton, Ontario
An Introduction to the Electronic Structure of Atoms and MoleculesProfessor of Chemistry / McMaster University / Hamilton, Ontario
|
We may use an extension of our simple system to illustrate
another important quantum mechanical result regarding energy levels. Suppose
we allow the electron to move on the x-y plane rather than just
along the x-axis. The motions along the x and y directions
will be independent of one another and the total energy of the system will
be given by the sum of the energy quantum for the motion along the x-axis
plus the energy quantum for motion along the y-axis. Two quantum numbers
will now be necessary, one to indicate the amount of energy along each
coordinate. We shall label these as nx
and ny. Let us assume that the motion
is confined to a length L along each axis, then:
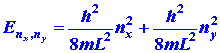
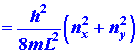 |
|
Since two dimensions (x and y) are now required to specify the position of the electron, the probability distribution P1,1(x,y) must be plotted in the third dimension. We may, however, still display P1,1(x,y) in a two-dimensional diagram in the form of a contour map (Fig. 2-7). All points in the x-y plane having the same value for the probability distribution P1,1(x,y) are joined by a line, a contour line. The values of the contours increase from the outermost to the innermost, and the electron, when in the level nx = ny = 1, is therefore most likely to be found in the central region of the x-y plane.
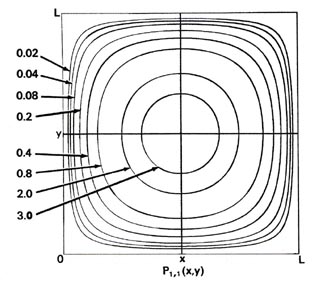
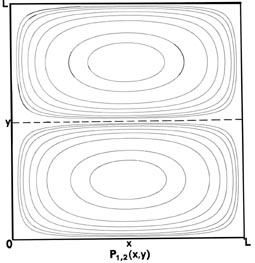
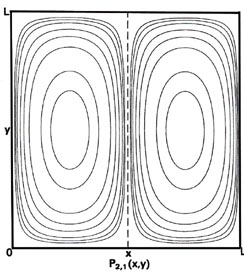
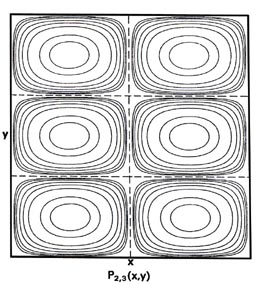
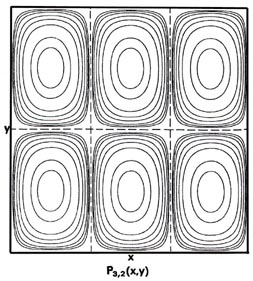
Fig. 2-7. Contour maps of the probability distributions Pnx, ny (x,y) for an electron moving on the x-y plane. The dashed lines represent the postion of nodes, lines along which the probability is zero. P1,2 (x,y) and P2,1 (x,y) are distributions for one doubly-degenerate level; P2,3 (x,y) and P3,2 (x,y) are examples of distributions for another degenerate level of still higher energy. The same contours are shown in each diagram and their values (in units of 4/L2) are indicated in the diagram for P1,1(x,y).
A plot of P1,1(x,y) along
either of the axes indicated in Fig.
2-7 (one parallel to the x-axis at y = L/2 and
the other parallel to the y-axis at x = L/2) is similar in
appearance to that for P1(x)
shown in Fig.
2-4. That is, for a fixed value of y, the contribution to P1,1(x,y)
from the motion along the y-axis is constant and
|
|
Now consider the possibility of nx
= 1 and ny = 2. Then
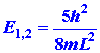 |
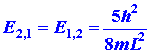 |
The concept of degeneracy in an energy level has important consequences in our study of the electronic structure of atoms.
 |
 |
 |
 |