Total Synthesis
Target oriented synthesis often reveals limitations in current methodologies, displaying the need for new reagents and useful synthetic strategies. For example, our recent synthesis and structural revision of Phomolide G and H demonstrate the utility of DualPhos, a readily accessible reagent for the preparation of latent alkenals. As well, the Amaryllidacea alkaloids (e.g. trans-dihydronarciclasine and trans-dihydrolicoricidine) have been the subject of significant synthetic efforts in out lab, given their potent anti-viral and anti-cancer activities. Our syntheses hinge on a novel organocatalytic [3+3] cycloaddition between an alkenal and azidoacetone. The discovery of this reaction has allowed us to obtain the target compounds in fewer steps and higher yield than previously reported syntheses. |
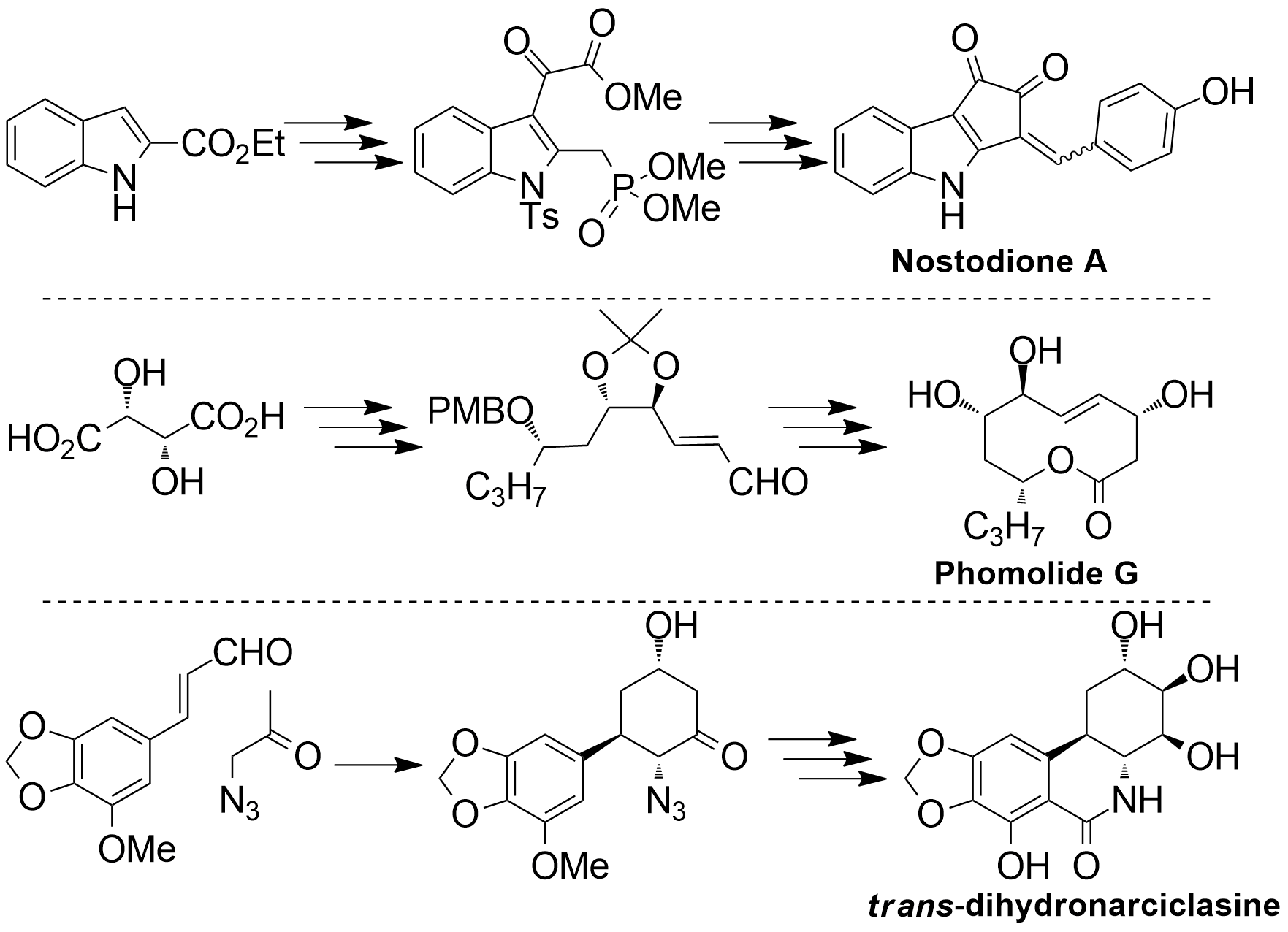 |
In line with our efforts to discover new anti-parasitic compounds, we developed a diversity oriented synthesis of Nostodione A, a cyanobacterial metabolite with structural similarity to known anti-parasitics. Our synthetic strategy allowed the creation of a mini-library of structural analogues, some of which demonstrated activity against T. gondii with IC50 values comparable to those of atovaquone. |
|
Relevant Articles Amaryllidacea Alkaloids Synthetic Approaches to the Naturally Occuring Anticancer Amaryllidaceae Alkaloids trans-dihydrolycoricidine and trans-dihydronarciclasine. C. Zepeda-Velázquez, J. McNulty*, Studies in Natural Products Chemistry, 53, in press (2017). Total Synthesis of the Natural Product (+)-trans-Dihydronarciclasine via an Asymmetric Organocatalytic [3+3]-Cycloaddition discovery of its potent anti-Zika Virus (ZIKV) Activity. O. Revu, C. Zepeda-Velazquez, A. Nielsen, J. McNulty*, R. H. Yolken, L. Jones-Brando, ChemistrySelect, 1, 5895-5899 (2016). Enantioselective Organocatalytic Michael-aldol Sequence to the Anticancer Natural Product (+)-trans-dihydrolycoricidine. J. McNulty*, C. Zepeda-Velazquez, Angew. Chem. Int. Ed. 53, 8450-8454 (2014). See also: Short Synthesis of a Wildflower Alkaloid, Chem. & Eng. News, July 7th, 2014, p. 28-29. See also: Synfacts, 10, 982 (2014). Phomolides G and H Total Enantioselective Synthesis of the Endophytic Fungal Polyketide Phomolide H and its Structural Revision. J. McNulty*, D. Mcleod, Eur. J. Org. Chem. In press, (2017). DualPhos: a versatile, chemoselective reagent for two-carbon aldehyde to latent alkenal homologation application in the total synthesis of phomolide G. D. Mcleod, J. McNulty*, Roy. Soc. Open Sci. 3, 160374 (2016). Enantioselective Total Synthesis of the Proposed Structure of the Endophytic Fungal Metabolite Phomolide G: Structural Revision and Unambiguous Stereochemical Assignment. J. McNulty*, D. McLeod, H. A. Jenkins, Eur. J. Org. Chem. 688-692 (2016). Nostodione A Synthesis of the cyanobacterial metabolite nostodione A, structural studies and potent antiparasitic activity against Toxoplasma gondii. J. McNulty*, K. Keskar, H.A. Jenkins, N. H. Werstiuk, C. Bordon, R.Yolken, L. Jones-Brando., Org. Biomolec. Chem., 13, 10015-10024 (2015). Total Synthesis of the Cyanobacterial Metabolite Nostodione A: Discovery of its Antiparasitic Activity Against Toxoplasma gondii. |
|