H2(g) + 1/2 O2(g)
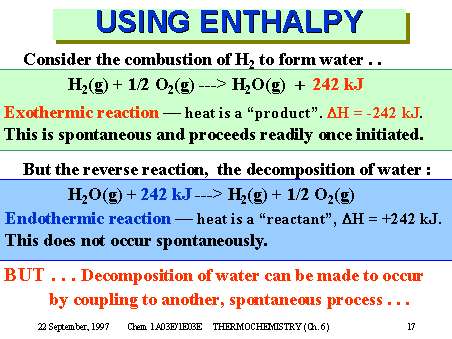
Endothermic reaction — heat is a “reactant”, DH = +242 kJ.
This does not occur spontaneously.
Consider the combustion of H2 to form water . .
H2(g) + 1/2 O2(g) ---> H2O(g) + 242 kJ
Exothermic reaction — heat is a “product”. DH = -242 kJ.
This is spontaneous and proceeds readily once initiated.
USING ENTHALPY
BUT . . . Decomposition of water can be made to occur
by coupling to another, spontaneous process . . .
-->