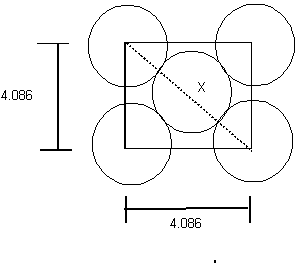

From Pythagorus' theorum, X = ![]() = (4.0862+4.0862)0.5
= (4.0862+4.0862)0.5
This give the hypotenuse, X, which represents 4 radii of the silver atom, the Radii is thus, 1.44 Å.
The volume: 4/3 r3 = 12.61 Å3 (Å3 = 10-24cm3), the volume = 1.26 x 10-23 cm3
There are 4 atoms in the unit cell. Each atoms occupies 12.61 Å3 x 4 atoms = 50.44 Å3
The unit cell volume = (4.086Å)3 = 68.22 Å3 Thus, in the cell 73.9% is occupied by Ag, 26.1% is empty space.
2a) Which would be more effective for cooling a glass of your favourite beverage: 30 g of 0C water of 30 g of 0 C ice?
The ice: in this case the 0 C water absorbs energy from the drink - the net temperature of the drink + water decreases AND the melting of the ice requires the enthalpy of fusion for 30 g of ice, which again absorbs heat from the drink.
b) What is the pressure of 8 g of CS2 (MW) = 80 g/mol if allowed to evaporate into a vacuum at 25 C in a vessel of volume of 8 L? What about 40 g in 5 L? The vapor pressure of CS2 at 25 C is 336 mmHg
8 g CS2 = 0.1 M, PV = nRT,
P atm (8L) = 0.1 x 0.082 L atm/mol K x 298 K; = .305 atm x 760 mmHg/atm = 232.1 mmHg
P = 232.1 mmHg (there is not enough CS2 to fill the vessel to its vapour pressure)
40g CS2 = 0.5 M
P = 1160 mmHg (BUT THIS CAN'T BE RIGHT. ONLY ENOUGH CS2 WILL EVAPORATE TO ESTABLISH THE Pv OF 336 mmHg.) The pressure is 336, thus only 0.144 moles of CS2 will evaporate, the rest (.356 mol) will stay condensed in the vessel.
3) The pH of a household ammonia solution is 11.50. What is the molarity of the solution?
Kb for NH3 = 1.8 x 10-5
From the pH, pOH = 2.5, therefore [OH-] is 3.2 x 10-3 M (convert pH to pOH, -antilog gives [OH-]).
Therefore, [NH4+] = [OH-]=3.2 x 10-3 M.
![]()
Kb = [NH4+][OH-]/[NH3] = 1.8 x 10-5
= (3.2 x 10-3)2/x - 3.2 x 10-3 Assume x >> 3.2 x 10-3
x = 0.57 M = [NH3], 0.57 is >> 3.2 x 10-3
4) Calculate the concentrations of all the products/reagents in a 0.10 M hypochlorous acid (HOCl). The Ka of HOCl is 3.5 x 10-8. You don't need to calculate the molarity of water.
![]()
Ka = [H3O+][-OCl]/[HOCl] = 3.5 x 10-8
Ka = x2/0.1-x = 3.5 x 10-8
assume 0.1 >> x
x = 5.9 x 10-5 (which is much << than 0.1)
The conc of [H3O+] and of [-OCl] is 5.9 x 10-5, the conc of HOCl = 0.1 - 5.9 x 10-5 = 0.1
5). A 0.083 M solution of a monoprotic acid (HA) is known to be 1.07% ionized. What is the pH of the solution? Calculate the Ka for this acid.
![]()
Ka = [A-][H3O+]/[HA]
since we know the acid is 1.07% ionized, we can solve for [H3O+]
0.0107 * 0.083 = [H3O+], pH = 3.05
Ka
Ka = (0.083 x 0.0107)2 / (0.083-0.0083x0.0107)
Ka = (8.8 x 10-4)2 / (0.083) = 9.6 x 10-6
6) Triethylamine and trimethylamines are both bases. Which one is stronger? Calculate the pOH, of a 0.015 M solution of each amine, respectively.
From the Kb's we immediately know which is the better base. The higher the number, the more the equilibrium lies to product (i.e., that is has done its thing as a base), the better the base. Triethylamine is a better base than trimethylamine. You can also see this from the pOH of the solutions of the two bases. Triethylamine generates more OH-
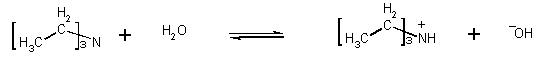
Kb = 5.2 x 10-4
= x2/0.015-x assume x << 0.015
x2 = 7.5 x 10-6
x = 2.74 x 10-3
but x is more than 5% of 0.015, so must solve quadratic. When you do, x = 2.5 x 10-3
pOH = 2.6
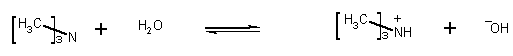
Kb = 7.4 x 10-5
7.4 x 10-5= x2/0.015-x assume x << 0.015
x2 = 1.11 x 10-6
x = 1.05 x 10-3
but x is more than 5% of 0.015, so must solve quadratic. When you do, x = 1.0 x 10-3
pOH = 3
Why is Et3N a better base? This is because an ethyl group donates electron density to the central N better than a methyl group. We will talk more about this during organic chemistry