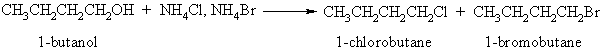
| McMaster University - Chem2O06 Lab Manual | 1997/98 |
Experiment 4. Part A: Nucleophilic Substitution and Gas Liquid Chromatography.
Nucleophilic Substitution
Reference: Ege, Chapters 4, 7
Do this Part in pairs, one person performing Part A-1 and the other Part A-2. Remember to record the name of your partner!
PART A-1
In this experiment no discussion is given, because the student is expected to develop his own explanation for the results of the experiment. Ammonium halides were chosen because both the chloride and bromide are soluble at the concentrations needed for the conversion:
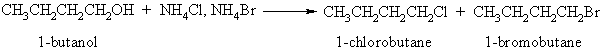
Attach the simple condenser and on the top of the condenser fit your take-off adaptor with a hollow glass stopper (B-19) in the outer joint (see Fig. 1), and a piece of rubber tubing leading to a trap for acidic gases.
Note: the mouth of the inverted funnel should be slightly tilted and just below the surface of the water). To ensure that your inverted funnel stays in the correct position, you should hold it in place with a clamp.
Commence heating the reaction mixture with a heating mantle placed directly on top of the magnetic stirrer and apply a fairly high voltage to the heating mantle. Watch carefully and cut back on the voltage as soon as you see the reaction mixture begin to boil. Control the heating rate so that the refluxing vapours rise no more than 1.5 - 2.5 cm up into the jacketed portion of the condenser.
Reflux the solution, with constant magnetic stirring, for about 1 hour.
Remove the heating mantle and the tube to the gas-trap and, using ice water, cool the reaction mixture to room temperature, add 15 mL ether and transfer the mixture to a separatory funnel. Shake the mixture, discard the aqueous layer and wash the organic layer, once with 15 mL of ice-cold water, and once with about 15 mL of 5% aqueous bicarbonate (CARE - pressure may build up). Transfer the organic layer to a 25 mL Erlenmeyer flask containing 2-3 g of anhydrous calcium chloride. Stopper the flask, and swirl occasionally for about 5 minutes. Then filter the liquid through a fluted filter paper (see Expt-1, Fig. 2) into a 25 mL round bottom flask.
Set up a distillation apparatus (see Expt-3, Fig. 5, but use a round bottomed flask attached to the receiver-adaptor, not a measuring cylinder) and distill off the ether using a beaker of hot water, until about one quarter your solution remains (you should have from 3-4 mL of liquid remaining). Insert a lightly greased stopper into the flask, and retain in your locker for glc analysis the next laboratory period.
Note 1: you can save some time by setting up your distillation apparatus and heating the beaker of hot water on the hot plate during the reaction reflux period.
Note 2: Be sure to seal your sample well. If it's not, your compound will completely evaporate during the intervening two weeks!
PART A-2
Carry out the procedure described in Part A-1, but use 2.6 g 2-methyl-2-butanol in place of 1-butanol. Prepare the acid solution as in Part A-1, adding your 1" magnetic stir bar and the ammonium salts to the clamped flask. Allow the mixture to stir for 3 - 5 min. when the majority of the ammonium salts should dissolve. If your mixture is not too hot (eg above 30 - 35oC) immediately add 2.6 g of 2-methyl-2-butanol slowly, and with stirring, to the mixture (cool if it appears to become too warm). If your mixture is still too warm after 3 - 5 min. cool it slightly with a cold water bath and then proceed as above.
When the addition is complete, attach the flask to the condenser and trap, and gently reflux the solution for 3/4 hour. Work-up as described in Part A-1. It is essential, if you wish to obtain good results in this experiment, to remove the bulk of the ether solvent by distillation (see Expt-3 Fig. 5, but use a round bottomed flask attached to the receiver-adaptor, not a measuring cylinder). Stopper your flask with a lightly greased ground glass stopper and retain in your locker for glc analysis the following laboratory period.
Experimental Notes
1. Be very careful inserting your thermometer into the glass-to-rubber adaptor; use a little glycerol as lubricant and grasp the thermometer close to the adaptor as you push it in.
2. When starting the water flow through the condenser do not turn the tap on full blast! A gentle flow will suffice.
Gas-Liquid Chromatography (Vapour Phase Chromatography)
The essential features of a gas chromatograph are shown below. The column, consisting of a long glass or metal tube and usually coiled so that it can be fitted into a compact oven, is filled with column packing. The packing is an inert support material, such as some form of Celite, coated with the stationary phase which is generally a viscous oil of low volatility. Silicone oils, Apiezon (hydrocarbon) greases, and polyesters of high molecular weight are commonly used for this purpose.
The column is mounted in an oven which can be accurately held at any temperature between room temperature and 250oC (usually). The carrier gas (mobile phase) is usually nitrogen (but sometimes argon, helium, or hydrogen) and passes continuously through the column throughout its operation.
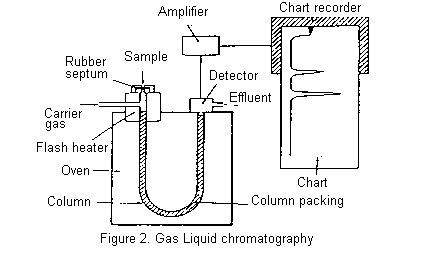
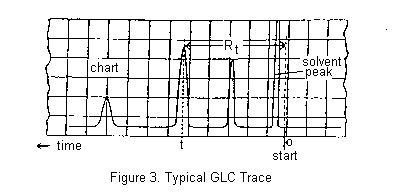
A small volume of the mixture to be separated is injected through a rubber septum into the column where it is vapourized, sometimes with the help of a flash heater. The components of the mixture partition themselves between the stationary and mobile phases and move through the column, ideally at different rates, separating into discrete bands. More volatile components are eluted before less volatile compounds, though secondary factors such as polarity may sometimes invert the expected order. The column effluent passes through the detector which senses the separated components as they are eluted and send an electric signal through an amplifier to a chart recorder. A typical chromatogram is shown below.
Procedure:
Your TA will organize a time for you and your partner to obtain chromatograms for both samples (Part A-1 and Part A-2). It is very important to make sure that your sample is diluted with 1-3 mL of ether immediately before glc analysis - the pure chlorides and bromides are too concentrated and the glc trace will go wildly off scale.
You should record the instrument parameters carefully: i.e. the type of column used, column temperature, the Helium flow rate, chart paper movement rate in cm/min., and the instrument manufacturer's name. All these data should be recorded on the chart paper (including your name, the date, and what you injected onto the column!) as well as in your Lab Notebook. Work out the retention time Rt for each of your peaks (see Figure 3) and label them, including the solvent peak. Measure the relative areas of the 1-chlorobutane and 1-bromobutane (or 2-chloro-2-methylbutane and 2-bromo-2-methylbutane) peaks obtained by the chromatographic separation of the mixture. Assume that the weight ratio of the components is equal to the ratio of the area of the peaks.
To be included in your lab book for this experiment:
1. The boiling points of 1-chlorobutane, 1-bromobutane, 2-chloro-2-methylbutane, and 2-bromo-2-methylbutane; indicate whether the glc retention times correlate with boiling point.
2. Comment on whether your results allow you to conclude which is the better nucleophile, chloride or bromide ion.
3. Comment on whether your results allow you to conclude anything regarding the mechanisms of these reactions. Identify any additional factors which might make this difficult.
| Go to: | Instructions for Printing this Document Experiment 4 - Main Page Part B: Properties of Alkanes & Alkenes Chem2O06 Home Page. |
14oct97; wjl