| McMaster University - Chem2O06 Lab Manual | 1997/98 |
Experiment 5. The Stereochemistry of Bromination. Preparation of meso-Stilbene Dibromide and its Conversion to Diphenylacetylene
Reference: Ege, Chapter 8
In this experiment, you will carry out the electrophilic bromination of E-1,2-diphenylethene (E-stilbene), and then convert the product into diphenylacetylene by a dehydrohalogenation reaction.
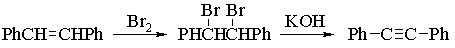
1. Addition of Bromine to E-Stilbene
Caution! Both bromine and acetic acid are noxious liquids which should be handled with extreme care. In case of spillage onto the skin, the affected area should be washed immediately with water. The bromination of E-stilbene MUST be performed in the fume hood. Safety glasses MUST (as always!) be worn.
Procedure: Weigh out 1 g of E-stilbene into a 125 mL Erlenmeyer flask, add 20 mL of acetic acid and dissolve the stilbene by heating gently on a hot plate in a fume cupboard. When all the stilbene has dissolved, add 4 mL of the solution of bromine in acetic acid provided (this contains 100 g of bromine in 300 mL of acetic acid) and continue heating the solution for 2-3 mins. Remove from the heat and cool under the tap. The product, which precipitates from the reaction mixture, should be collected by filtration on a Buchner funnel and washed with two portions of cold methanol. Dry the sample thoroughly on a filter paper, and determine the yield. Keep a small sample aside to determine its melting point when you do the melting point of your diphenylacetylene.
2. Conversion of meso-Stilbene Dibromide to Diphenylacetylene
Alkynes are commonly prepared by the dehydrohalogenation of 1,2-dihalides under basic conditions. Normally, a very strong base such as sodamide (NaNH2) is necessary, but treatment of meso-stilbene dibromide with potassium hydroxide leads to the formation of diphenylacetylene in high yield.
Procedure: Place 1 g of meso-stilbene dibromide, 8 pellets of potassium hydroxide (ca. 0.6 g) and 4 mL of triethylene glycol into a Pyrex test tube. Your T.A. will have set up at the beginning of the period a sand bath which will be preheated to 180-195 oC. Clamp your test tube in a vertical position and insert it into the sand up to the level of the reaction mixture. Allow the reaction mixture to heat for 3-4 minutes and then stir it gently with a glass rod. Continue the heating for a further 20-25 minutes and stir every 5-10 minutes (it is important that the KOH is uniformly distributed). Remove the test tube and allow it to cool to room temperature. Add 20 mL of cold water while thoroughly mixing the sticky mass with a spatula and filter off the precipitate on a Buchner funnel. Wash the crystals with water. Recrystallise the product from ethanol* (DO NOT WASH THE FINAL CRYSTALS!) and determine the yield and melting point. (Place your diphenylacetylene in the jar provided.)
Be sure to comment on any differences between the melting point of your products in parts 1 and 2 and those of the pure compounds:
meso-1,2-dibromo-1,2-diphenylethane: m.p. 241 oC (dec)
d,l-1,2-dibromo-1,2-diphenylethane: m.p. ___ oC
diphenylacetylene: m.p. 61 oC
* If you have forgotten how to recrystallize a solid compound, review the section on recrystallization in Experiment 1. Hint: only about 4 mLs of ethanol is required.
If you have time, clean your equipment for Experiment 6.
Before coming to the lab, you should be able to answer the following questions:
1. Draw the products of bromination of Z- and E-3-hexene. Specify which (if any) of these products is optically active. Are any of the structures identical? Explain.
2. Write a mechanism for the addition of bromine to E-stilbene. Draw a perspective formula of the final product.
3. E-Stilbene on bromination gives meso-stilbene dibromide. What product(s) would be formed by the bromination of Z-stilbene? Use perspective formulas to illustrate your answer. Assign absolute configurations to all stereocenters in these molecules using the Cahn-Ingold-Prelog convention.
4. Why, when sodamide in liquid ammonia is normally used for the dehydrohalogenation of dihalides to acetylenes, may a weaker base such as KOH be used to prepare 1,2-diphenylacetylene?
| Go to: | Instructions for Printing this Document Chem2O06 Home Page. |
25oct97; wjl