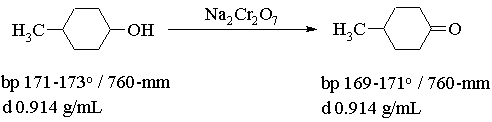
| McMaster University - Chem2O06 Lab Manual | 1997/98 |
Experiment 6. Dichromate Oxidation of 4-Methylcyclohexanol and Infrared Spectroscopy
References: Ege, Chapter 10,12; Expt 1 (Extraction Techniques)
In this experiment you will prepare a sample of 4-methylcyclohexanone by the oxidation of a mixture of cis-and trans-4-methylcyclohexanol and then characterize the product by its infra-red spectrum. The experiment will be run over two days in order that everyone may run an IR spectrum of their product.
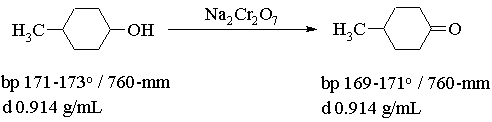
Oxidation is a very common reaction in organic chemistry and chromium (VI) compounds are especially useful as oxidizing agents. At the end of the oxidation the chromium is usually reduced to chromium (III). Recall that primary alcohols will oxidize in two stages, firstly to aldehydes and then to carboxylic acids:
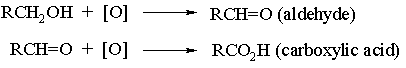
The second oxidation step is normally very facile and either special oxidants (e.g. pyridine chlorochromate, PCC), or special experimental conditions must be employed (eg rapid removal of the aldehyde from the oxidant by distillation) in order to obtain aldehydes in good yield. Secondary alcohols are more straightforward and, provided excessive heat is not used, they will oxidize smoothly to ketones:
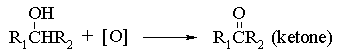
Tertiary alcohols are stable to oxidizing agents at normal temperatures. However, heating in the presence of acidic oxidizing agents will cause elimination and thence oxidation of the alkene.
Procedure. Weigh 2.0 g of 4-methylcyclohexanol (a mixture of cis and trans isomers) into a 125 mL Erlenmeyer flask and add 10 mL of diethyl ether (CAUTION - NO FLAMES). Using an ice bath to maintain a temperature of about 0o and a magnetic stirrer to GENTLY stir the solution, add 10.0 mL of 0.67 M acidified sodium dichromate (Caution: oxidizing agent! use gloves) dropwise from a pipet over a period of about 10 minutes. Continue stirring for 10 minutes after the last addition of dichromate. The mixture should change colour and may become very dark.
Transfer the mixture to your large separatory funnel, rinse the 125 mL Erlenmeyer flask with another 15 mL of ether and separate the phases (first extraction). Take the aqueous portion and extract it two more times with 10 mL portions of ether. COMBINE all three ether portions! Discard the aqueous layer in the WASTE INORGANIC.
[Note: In the first two extractions the phases are VERY difficult to differentiate. To better see the meniscus in the first extraction, drain off 12 to 14 mL of the aqueous layer and add 5 mL H2O and re-shake gently with the ether. Also use the light provided in the fumehood. Note that the viscosity of the two phases are different and if you are careful you can tell when the water phase has drained out. Because of different surface tensions, the water phase tends to stick to one side of the drain spout whereas the ether flows out of all sides.]
Wash the combined ether extracts with 10 mL each of: water, 5% sodium bicarbonate, water, and saturated sodium chloride. Add approximately 2 g of anhydrous sodium sulphate to the ether extract to dry it, and then filter it (gravity, see Expt-1, Fig-1) into a DRY, pre-weighed 125 mL Erlenmeyer flask. Remove the excess ether by allowing it to boil off (add a boiling chip!) using a steam bath in a fume HOOD; do not use a hot plate!. Determine the yield of the product.
Ensure that your sample of 4-methylcyclohexanone is DRY and free of particulate matter before you attempt to determine the IR spectrum.
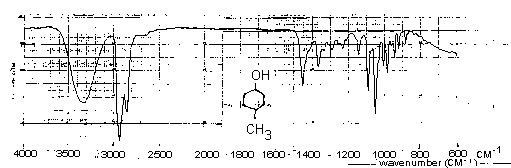
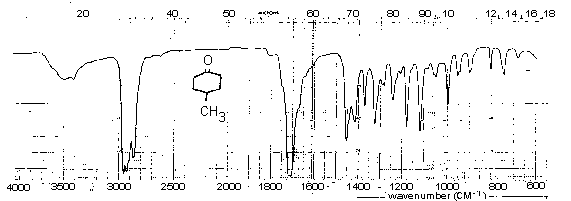
See your demonstrator about recording the infrared spectrum of your product. Spectra of authentic samples of 4-methylcyclohexanol and 4-methylcyclohexanone are provided below. Assign the major bands of these spectra and those in the spectrum of your product. Comment on any differences between the spectrum of the ketone from your synthesis and the one supplied.
ARRANGE A 5 MINUTE TIME SLOT IN ORDER TO OBTAIN AN IR SPECTRUM. KEEP THE REMAINDER OF YOUR KETONE FOR USE LATER IN A LATER EXPERIMENT.
Recording an Infrared Spectrum
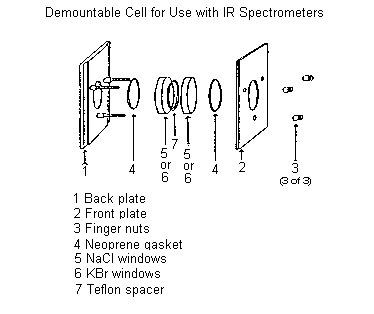
It is important to obtain a "good" infrared spectrum; i.e., a spectrum showing both the strong and weak absorptions of the compound of interest. Thus, the amount of compound must be adjusted so that the strongest peaks give transmittance values of 10-30%. If too much compound is used, peaks will go off-scale (transmittance = 0%); if too little material is used, weak absorptions will not be observed. ONE drop of 4-methylcyclohexanone from a Pasteur pipette on one of the KCl discs of the demountable cell should be sufficient to obtain a good spectrum. The cell should be mounted without a spacer (see below). Do not over-tighten the finger nuts!
Infrared Spectrum of 4-Methylcyclohexanol
Infrared Spectrum of 4-Methylcyclohexanone
| Go to: | Instructions for Printing this Document Chem2O06 Home Page. |
25oct97; wjl