
Chem2O6
- 1997/98| Problem Set #2 | September 19, 1997 |
1. Write the following condensed formulas as Lewis structures:
(i) (CH3)3SO+ (ii) CH2N2 (iii) CH3NHCONH2 (iv) CH3CH2N3 (v) CHCCHNCH3
2. Write resonance structures for the following, indicating major and minor contributors (C6H5 is a phenyl ring).
(i) [CH3CHOCHCH2]+ (ii) [CH3CH2OCHCHCH2]+ (iii) [C6H5CH2]- (iv) [CH3COCH2]-
(v) [CH2CHCHCHCH2]+ (vi) [C6H5CHNH2]+
(vii) [CH2CHCH2]
3. Draw a molecular orbital diagram for carbon tetrachloride (CCl4), using sp3 hybrid atomic orbitals for carbon and a 3p atomic orbital for each of the chlorines. Fill the MO's with the eight electrons involved in sigma-bonding in the molecule. Draw pictorial representations of one of the bonding and one of the antibonding MO's.
(a) Irradiation of a solution of CCl4 in cyclohexane C6H12) with UV light results in the formation of the following products:
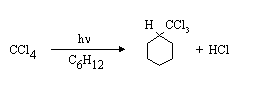
Suggest a mechanism for the reaction, and explain what the role of the UV light is, using your MO diagram for assistance.
(b) Reduction of carbon tetrachloride with sodium metal leads to the formation of hexachloroethane (C2Cl6) and sodium chloride. Suggest a mechanism for this reaction, again using MO diagrams to help explain why the reaction occurs.
4. Draw a molecular orbital diagram for water (H2O), using two hydrogen 1s atomic orbitals and four oxygen sp3 hybrid orbitals. Fill the MO's with the eight electrons.
Why would it be incorrect to construct the MO's using sp hybrid atomic orbitals for the O-H bonds, and two pure p-orbitals for the lone pairs?
| Go to: | Instructions for Printing this Document Answers Chem2O6 Problem Sets & Answers Chem2O6 Home Page. |
19sep97; wjl