Chem2O6
- 1997/98| Problem Set #3 | ANSWERS | October 2, 1997 |
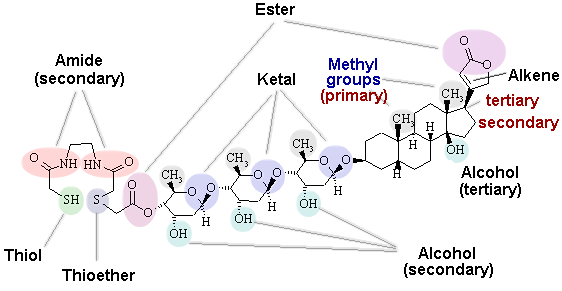
1. The digitoxin derivative shown below is a metal chelating agent that is specific for ATPase and has potential use in radio-imaging.
(i) Identify the functional groups present in this compound.
(ii) Identify a methyl group, and a primary, secondary, or tertiary carbon
(if there are any present).
(iii) Identify a primary, secondary, or tertiary alcohol (if any are present).
(iv) Identify a primary, secondary, or tertiary amide (if any are present).
Answer:

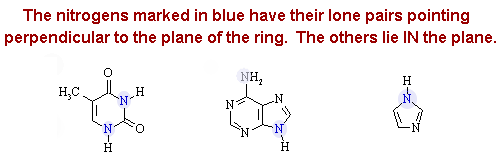
2. Consider the following three organic molecules:
(a) Give the hybridization of each of the atoms (excluding hydrogens) in each molecule.
Answer: The lone pairs on EACH of the NH nitrogens
in all three structures can overlap with either a C=O, C=C, or C=N ![]() system, and are therefore sp2 hybridized. The only atom
which is sp3 hybridized is the methyl carbon in Thymine.
system, and are therefore sp2 hybridized. The only atom
which is sp3 hybridized is the methyl carbon in Thymine.
(b) In which direction do the lone pairs on the N atoms in the three structures point relative to the plane of the rings?
Answer:
(c) A cyclic array of 6 ![]() electrons (or 6
electrons in overlapping 2p orbitals is said to be aromatic:
electrons (or 6
electrons in overlapping 2p orbitals is said to be aromatic:
(i) Would you expect thymine, adenine, or imidazole to be
aromatic? If so, what resonance structures would you have to write in order to obtain 6 ![]() electrons in a cycle?
electrons in a cycle?
Answer: While only adenine has a six-membered ring with 3
conjugated double bonds (like benzene), all three compounds have some degree of
aromatic character. If we consider the Lewis structures shown below, and count the lone
pairs on N atoms which are pointing perpendicular to the planes of the rings as
contributing to the cyclic array of 2p electrons, we see that both thymine and imidazole
are aromatic. Adenine is like naphthalene, an aromatic compound with 10 ![]() electrons in a cyclic array of 2p orbitals. The
general rule for aromatic compounds is Huckel's Rule, which states that any cyclic
array of 4n+2 (where n is any integer)
electrons in a cyclic array of 2p orbitals. The
general rule for aromatic compounds is Huckel's Rule, which states that any cyclic
array of 4n+2 (where n is any integer) ![]() electrons
in p type atomic orbitals will have special stability.
electrons
in p type atomic orbitals will have special stability.
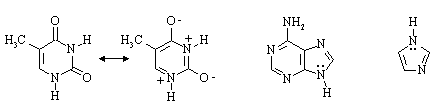
(ii) The first sites of protonation of adenine by a strong acid (e.g. HCl) are N(1), N(3), and N(7), NOT the NH2 group as one might expect. Write one or more resonance structures that would account for this.
Answer: The numbering system in adenine was omitted (sorry!); it's given below:
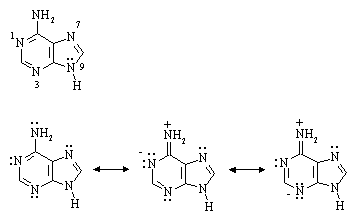
As shown by the two charge-separated resonance structures above, the lone pair on the NH2 nitrogen can delocalize into the ring, giving it slight positive charge and N1 and N3 slight negative charge. This means that the NH2 lone pair is less available for protonation by an acid than it would otherwise be. The same arguments can be applied to the lone pair on N9 (try it).
3. Show the direction (LHS or RHS) that you would expect the following equilibria to lie:
(i) CH3C N + C6H5-
N + C6H5- 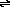 [CH2C
[CH2C N]- + C6H6
N]- + C6H6
Answer: RHS. Acetonitrile (pKa 25) is a stronger acid than benzene (pKa 43) and will therefore protonate the benzene conjugate base, phenyl anion.
(ii) CH2CH2 + OH- 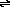 CH2CH-
+ H2O
CH2CH-
+ H2O
Answer: LHS. Water (pKa 115.7) is a much stronger acid than ethene (pKa 36) and will protonate the conjugate base, ethenyl anion.
(iii) OH- + CH3NH2 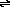 CH3NH-
+ H2O
CH3NH-
+ H2O
Answer: LHS. Water (pKa 15.7) is a much stronger acid than the N-H proton of methylamine ((pKa ~35) and will therefore protonate the methylamide anion.
(iv) CH3SH + CH3- 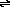 CH3S-
+ CH4
CH3S-
+ CH4
Answer: RHS. Mercaptomethane (CH3SH; pKa ~10) is a very much stronger acid than methane (pKa ~50), and will thus protonate the methyl anion.
(v) CH3CH2O- + C6H5COOH 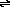 CH3CH2OH + C6H5COO-
CH3CH2OH + C6H5COO-
Answer: RHS. Benzoic acid (pKa 4.2) is stronger acid than ethanol (pKa 15.9), and will protonate ethoxide anion.
(vi) (C2H5)2NH2+ + HCO2-
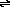 (C2H5)2NH + HCO2H
(C2H5)2NH + HCO2H
Answer: LHS. Formic acid (pKa 3.75) is a stronger acid than the diethylammonium cation (pKa ~11), and will thus protonate diethylamine.
4. The following equilibria all lie on the right hand side of the equation:
NH2- + CH3COOCH3 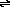 NH3 + -CH2COOCH3
NH3 + -CH2COOCH3
-CH2COOCH3 + CH3COCH3
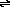 CH3COOCH3 + -CH2COCH3
CH3COOCH3 + -CH2COCH3
-CH2COCH3 + CH3OH 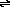 CH3COCH3 + CH3O-
CH3COCH3 + CH3O-
CH3O- + CH3NO2 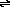 CH3OH + -CH2NO2
CH3OH + -CH2NO2
(i) Identify which compounds are weaker acids than methanol.
Answer: Methanol is able to donate a proton to acetone anion (CH3COCH2-) and is therefore a stronger acid than acetone (2-propanone). Likewise, it is a stronger acid than methyl acetate (because acetone is a stronger acid than methyl acetate) and ammonia.
(ii) Identify which compounds are weaker bases than methoxide ion.
Answer: Methoxide ion is involved in only one equilibrium which lies on the right (the last one); thus methoxide anion is a stronger base than nitromethane anion (-CH2NO2). All the others are stronger bases than methoxide anion.
(iii) Identify which compounds are weaker acids than acetone (2-propanone).
Answer: From the arguments in (i) above, 2-propanone is a stronger acid than methyl acetate and ammonia.
(iv) Identify which compounds are weaker bases than acetone anion.
Answer: 2-propanone anion is a stronger base than methoxide anion and nitromethane anion.
(v) Identify which is the strongest acid and which is the strongest base.
Answer: The strongest acid is nitromethane. The strongest base is amide anion (NH2-).
| Go to: | Instructions for Printing this Document Chem2O6 Problem Sets & Answers Chem2O6 Home Page. |
02oct97; wjl