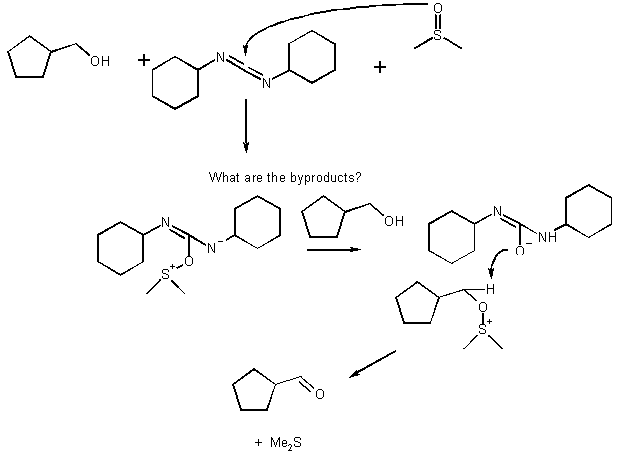

2) Suggest how to carry out the following transformations. You should use reagents that will be selective (i.e., regioselective, diastereoselective, use protecting groups if necessary). Each transformation may take a few steps.
In all cases, the product is on the right hand side!
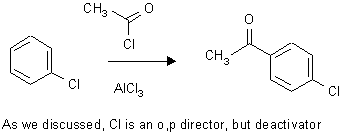
a) 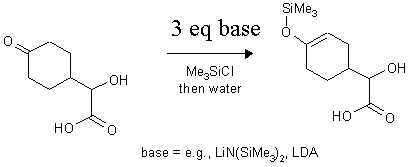
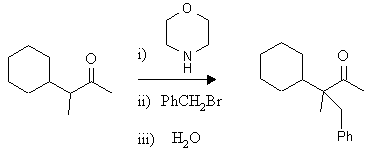
b) 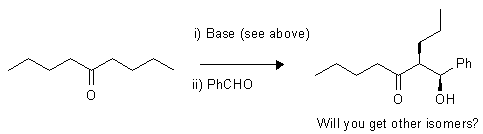
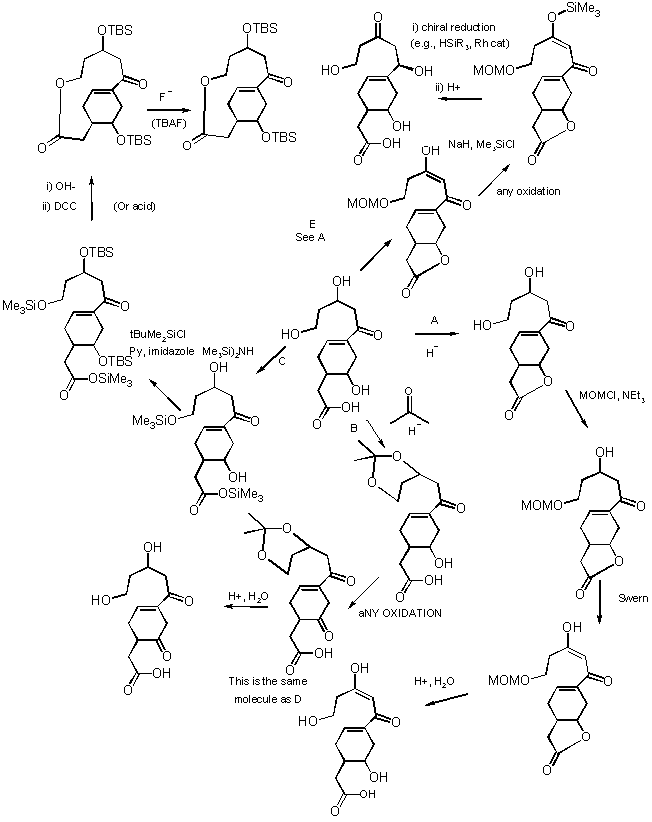
3) Suggest 2 different routes to synthesizing this compound (i.e., 2 different initial bond disconnections). The starting material may be any size but can only contain 2 functional groups. Each route should only involve 1-3 steps.
Depending on the way you number the compound, the normal or abnormal chemistry reveals itself. Some sample disconnections are given.
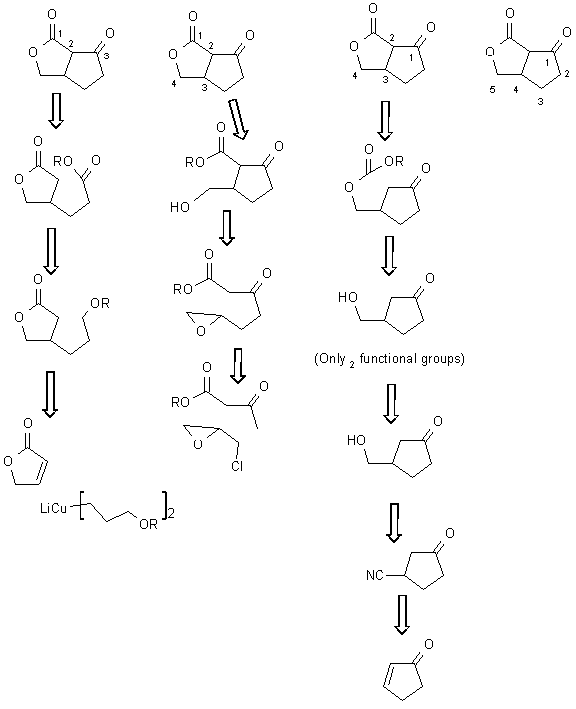
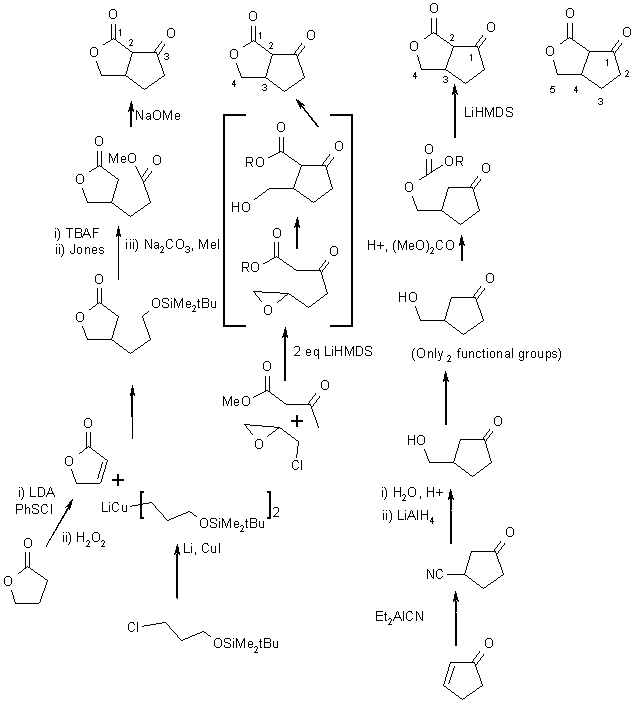
And their syntheses:
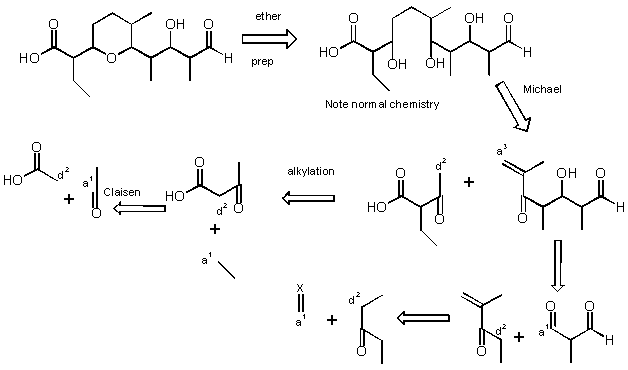
4). Show a disconnection which is reasonable for this molecule, which is a fragment in a compound called Salinomycin (no need for specific reactions, for each disconnection show acceptor (a) and donor (d). For the purposes of this course, in a Diels-Alder reaction, the diene is the donor, the dienophile is the acceptor). (Hint: if you open the ring at the oxygen in the first disconnection life will get easier).
Here is ONE of many possible solutions
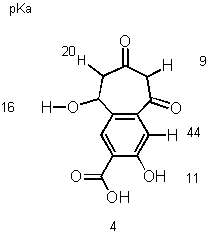
5). Show the 5 most acidic protons on the molecule and give the approximate pKa values for each.
6). Suggest how to undertake the following conversions. (Hint: how could you protect a diol?) In all these reactions, the trick is to have selectivity by protecting one or another of the groups.