| McMaster University - Chem2OB3 Lab Manual |
Experiment 1b. NMR Spectra of Carvone and Questions
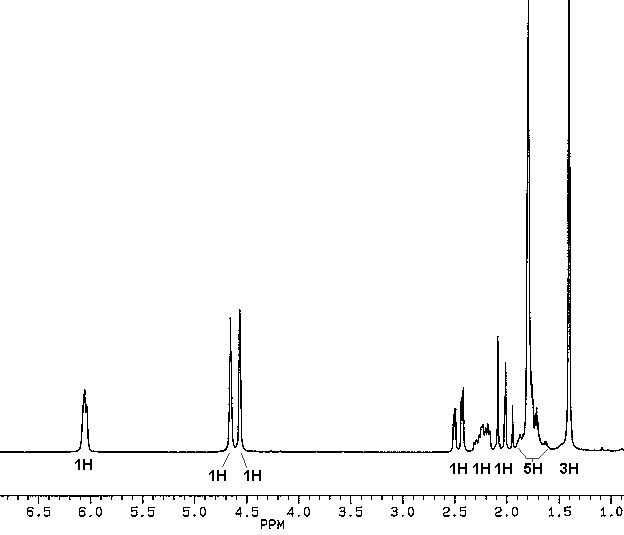
The 1H and 13C NMR spectra of (-)-carvone are shown below.
|
|
QUESTIONS
1. Interpret the IR spectra of carvone and limonene, and the 13C NMR spectrum of (-)-carvone. Identify the signals due to the vinylic protons and the methyl protons in the 1H NMR spectrum, and explain why the five aliphatic ring-protons give rise to such a complicated pattern in the
d1.5-2.6 region. How many non-equivalent hydrogens does this molecule possess?2. Identify the chiral centers in
a-phellandrene, b-phellandrene, and limonene.3. Explain how carvone fits the isoprene rule.
4. Using the Cahn-Ingold-Prelog sequence rules, assign priorities to the groups around the chiral carbon in carvone. Draw structural formulas for (+)- and (-)-carvone with the molecules oriented in the correct position to show the R and S configurations.
5. Explain why limonene elutes from the column before either (+)- or (-)-carvone.
6. Explain why the retention times for both carvone isomers are the same.
| Go to: | Instructions for Printing this Document Chem2OB3 Home Page. |
23dec99; jp