| McMaster University - Chem2OB3 Lab Manual | 1999/2000 |
Gas Chromatography
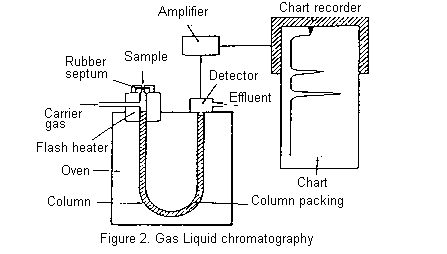
The essential features of a gas chromatograph are shown below. The column, consisting of a long glass or metal tube and usually coiled so that it can be fitted into a compact oven, is filled with column packing. The packing is an inert support material, such as some form of Celite, coated with the stationary phase which is generally a viscous oil of low volatility. Silicone oils, Apiezon (hydrocarbon) greases, and polyesters of high molecular weight are commonly used for this purpose.
The column is mounted in an oven which can be accurately held at any temperature between room temperature and 250oC (usually). The carrier gas (mobile phase) is usually nitrogen (but sometimes argon, helium, or hydrogen) and passes continuously through the column throughout its operation.

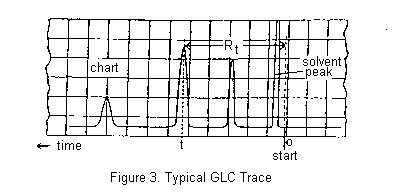
A small volume of the mixture to be separated is injected through a rubber septum into the column where it is vapourized, usually with the help of a flash heater. The components of the mixture partition themselves between the stationary and mobile phases and move through the column, ideally at different rates, separating into discrete bands. More volatile components are eluted before less volatile compounds, though secondary factors such as polarity may sometimes invert the expected order. The column effluent passes through the detector which senses the separated components as they are eluted and send an electric signal through an amplifier to a chart recorder. A typical chromatogram is shown below.
| Go to: | Instructions for Printing this Document Chem2OB3 Lab Manual Chem2OB3 Home Page. |
23dec98; jp