
| McMaster University - Chem2OB3 Lab Manual | 2001 |
Experiment 1. The Isolation of Natural Products
This two-part experiment will illustrate two of the techniques used for the isolation of organic compounds from natural sources - steam distillation and preparative gas chromatography. It will also give the student experience in infrared spectroscopy. Both parts of the lab will be carried out during the lab period.
Due to a limited number of gas chromatographs, some students may have to carry out Part B during Experiment 2.
Prelab Questions
(a) What is the boiling point of eugenol?
(b) The temperature of the distillation is about 100oC, the boiling point of water. Explain why eugenol is removed from the flask during the Steam distillation.
(c) The material recovered in the steam distillation contains mostly eugenol but it also has other components such as Caryophyllene. (See start of description of this experiment). Look up the properties of phenols such as eugenol in Browns, Foote and suggest a chemical method for separating eugenol and caryophyyllene.
Part A. Isolation of Essential Oils from Spices. The Technique of Steam Distillation.
Experiment 1a. Essential Oils from Spices: Oil of Cloves
The technique of steam distillation allows the separation of volatile components from non-volatile materials without the need for raising the temperature of the distillation above 100 oC. It provides a method for the isolation of natural products such as essential oils, which tend to be prone to decomposition at elevated temperatures. Essential oils are volatile compounds responsible for the aromas commonly associated with many plants.
The essential oils from cloves (from Eugenia caryophyllata) is rich in eugenol (4-allyl-2-methoxyphenol; bp 250 oC), which is one of a class of compounds known as phenols, which are compounds containing an hydroxy-substituted benzene ring. Caryophyllene is also present in relatively small amounts, along with other terpenes.

In order to complete the distillation in a reasonable length of time, it will be necessary to boil the mixture as rapidly as possible without allowing the boiling mixture to rise above the neck of the distillation head. This will require that you work with very careful attention during the distillation procedure. Once the distillation is complete, you will isolate the product by extraction and carry out a gas chromatographic analysis and separation.
Procedure
In this procedure we will be weorking at a somewhat larger scale in order to facilitate the steam distillation.
Weigh out approximately 2 grams of cloves and place them in a 100 ml roung bottom flask. Add about 45 ml of water and several boiling chips and let the flask stand while you assemble the rest of the equipment.
Set up the flask for distillation using a distillation head, thermometer, condenser, receiver, adapter and a heating mantle. Pleace a suitable Erlenmeyer flask to collect the distilate..
After the cloves have soaked in water for 15 minutes you should start to heat the flask and rapidly distill about 25 ml of the water. At this point add a further 25 ml of hot water to the distillation flask and then rapidly distill a further 25 ml. Record the temperature of the distillation.
Transger the distillate to a separating funnel. Wash the Erlenmeyer with 5 ml of dichoromethane and then add this dichloromethane to the separating funnel. Shale the contents of the separating funnel gently, allow the layers to separate, and remove the bottom layer containing the eugenol. Dry the dichloromethane layer using anhydrous sodium sulphate.
Weigh a vial and filter into it the dichloromethane solution using a gravity filtration (small filter paper or a glass-wool plug) to remove the sodium sukphate. Allow the dichloromethane to evaporate in the hood using a gentle heat from a steam bath. This can be done by placing the vial in the beaker of water which is then placed on the steam bath. Be careful not to overheat the vial as eugenol is volatile and can easily be lost. Record the weight of your recovered eugenol (plus other minor components. Calculate the %wt recoveery based on the starting weight of cloves you used.
Obtain the infrared spectrum of the oil. As it is a liquid you should be able to do this using the salt plates. Include the IR spectrum in your lab report. Assign the major peaks. Is there any evidence of dichloromethane in your spectrum?
Obtain the H NMR spectrum of eugenol from the web site and include a copy in your report. Assign the various resonances in the NMR spectrum.
Part B. Isolation of the Odorific Components of Essential Oils. Preparative Gas Chromatography.
Experiment 1b. Isolation of Essential Oils by Preparative Gas Chromatography
In this experiment, we isolate (+)-carvone from caraway seed oil or (-)-carvone from spearmint oil, using preparative gas chromatography. (+)- and (-)-carvone are enantiomers, and their odors are distinctly different from each other. This is to be expected, since the human body distinguishes odor by the interaction of molecules with odor receptors in the nose, which are chiral. The phenomenon in which a chiral receptor interacts differently with each of the enantiomers of a chiral compound is called chiral recognition. Another example of chiral recognition can be found in the toxicity of the two carvone isomers; the toxicity of (+)-carvone in rats is 400 times greater than that of (-)-carvone.
With the exception of their optical rotations, the physical properties of enantiomers are identical. Thus, we predict that the boiling points, refractive indices, gas chromatographic retention times, infrared and nuclear magnetic resonance spectra of (+)- and (-)-carvone should all be identical. The only difference in properties that one should observe are the odors and the signs of optical rotation in a polarimeter.
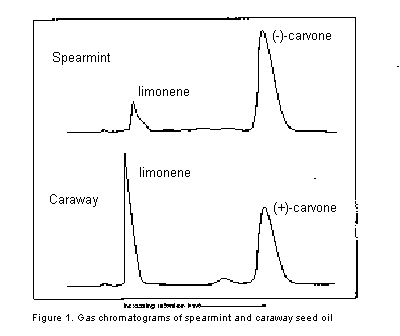
Caraway seed oil contains mainly limonene and (+)-carvone. The (+)-carvone (bp 230 oC) can easily be separated from the lower-boiling limonene (bp 177 oC) by gas chromatography, as illustrated in the GC-traces shown below in Figure 1. Using gas collection tubes, the two major components of the natural oil can be collected separately as they elute from the column. Spearmint oil contains mainly (-)-carvone, with a smaller amount of limonene and the isomeric terpenes, a- and b-phellandrene. The gas chromatogram of this oil is also shown in Figure 1. In this case, while it is easy to collect a pure sample of the carvone, limonene cannot be collected in pure form because it will be contaminated by the other terpenes, which have similar boiling points.
Your instructor will either assign you spearnint or caraway oil, or have you choose one of the oils. You will also be given instructions on which optional procedures, if any, you are to perform. Your instructor may have you isolate both the carvone and limonene components or only carvone. Avoid contact with (+)-carvone, as it is very toxic.
Gas Chromatography
In this experiment, you will carry out all aspects of the gas chromatographic separation yourself. The most critical part of the operation is the injection procedure - it is important that once you have pierced the injector with the syringe containing the mixture to be separated, you inject the mixture rapidly. Your T.A. will demonstrate the correct procedure for you; practice on your own several times with the small containers provided in the GC room, using the ethanol solutions provided, until you are confident that you can do it properly.
Inject 50-
mL of caraway-seed or spearmint oil onto the gas chromatography column. Just before a component of the oil (limonene or carvone) elutes from the column, install a gas collection tube at the exit port. To determine when to connect the gas collection tube, refer to the chromatograms on the wall of the GC room. These chromatograms have been run on the same instrument you are using under the same conditions. Ideally, you should connect the gas collection tube just before the limonene or carvone elutes from the column and remove the tube as soon as all the component has been collected, but before any other compound begins to elute from the column. This can be accomplished most easily by watching the recorder as your sample passes through the column. The collection tube is connected (if possible) just before a peak is produced, or as soon as a deflection in the pen is observed. When the pen has returned to the base line, the gas collection tube is removed.This procedure is relatively easy for collecting the carvone component of both oils and for collecting limonene in caraway-seed oil. Because of the presence of several terpenes in spearmint oil, it is somewhat more difficult to isolate a pure sample of limonene from spearmint oil (see chromatogram in figure). In this case, you must try to collect only the limonene component and not any other compounds, such as the terpene which produces a shoulder on the limonene peak in the chromatogram for spearmint oil.
After collecting the samples, insert the ground joint of the collection tube into a 0.1 mL conical vial, using an O-ring and cap to fasten the two pieces together securely. Insert the top of the collection tube through the hole in the rubber septum cap and place this assembly into a centrifuge tube. Put cotton on the bottom of the tube to prevent breakage. Balance the centrifuge by placing a tube of equal weight on the opposite side. During centrifugation, the sample is forced into the bottom of the conical vial.
Disassemble the apparatus, cap the vial, and perform the analyses described below.
ANALYSIS OF THE CARVONES
The samples obtained by gas chromatography and centrifugation should be analyzed by the methods below. Compare your results with those obtained by someone who used a different oil. In addition, measure the optical rotations of the commercial samples of (+)-carvone and (-)-carvone.
Gas Chromatography. Determine the retention times of the components. Calculate the percentage composition of the carvone sample by the triangulation method. In this method, a GC peak is approximated as a triangle, the area of which is proportional to the amount of material detected. The area of the peak is approximately equal to ½ X (peak height) X (peak width at half-height).
Infrared Spectroscopy. Obtain the infrared spectrum of the (-)-carvone sample from spearmint or of the (+)-carvone sample from caraway. Also obtain the infrared spectrum of the (+)-limonene, which is found in both oils. Determine both spectra on neat samples. If absolutely necessary, you may add one very small drop of nujol to the sample and mix it thoroughly before running the spectrum. The spectrum will contain extra C-H absorptions at around 3000 cm-1, however, as nujol (a.k.a. mineral oil) is a mixture of long chain aliphatic hydrocarbons.
Go to: Carvone NMR Spectra and Questions
| Go to: | Instructions for Printing this Document Chem2OB3 Home Page. |
December 19, 2000