An Introduction to the Electronic Structure of Atoms and Molecules
Professor of Chemistry / McMaster University / Hamilton, Ontario
An Introduction to the Electronic Structure of Atoms and MoleculesProfessor of Chemistry / McMaster University / Hamilton, Ontario
|
The study of the hydrogen atom is more complicated than our previous
example of an electron confined to move on a line. Not only does the motion
of the electron occur in three dimensions but there is also a force acting
on the electron. This force, the electrostatic force of attraction, is
responsible for holding the atom together. The magnitude of this force
is given by the product of the nuclear and electronic charges divided by
the square of the distance between them. In the previous example of an
electron confined to move on a line, the total energy was entirely kinetic
in origin since there were no forces acting on the electron. In the hydrogen
atom however, the energy of the electron, because of the force exerted
on it by the nucleus, will consist of a potential energy (one which depends
on the position of the electron relative to the nucleus) as well as a kinetic
energy. The potential energy arising from the force of attraction between
the nucleus and the electron is:
|
|
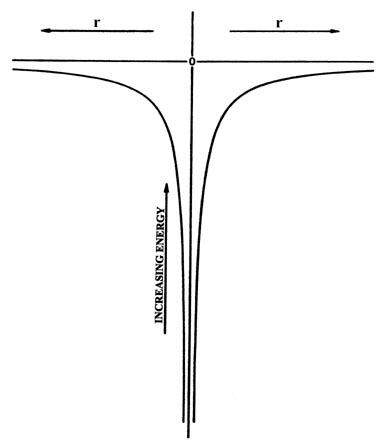
The potential energy is zero when the two particles are very far apart (r = ¥ ), and equals minus infinity when r equals zero. We shall take the energy for r = ¥ as our zero of energy. Every energy will be measured relative to this value. When a stable atom is formed, the electron is attracted to the nucleus, r is less than infinity, and the energy will be negative. A negative value for the energy implies that energy must be supplied to the system if the electron is to overcome the attractive force of the nucleus and escape from the atom. The electron has again "fallen into a potential well." However, the shape of the well is no longer a simple square one as previously considered for an electron confined to move on a line, but has the shape shown in Fig. 3-1. This shape is a consequence of there being a force acting on the electron and hence a potential energy contribution which depends on the distance between the two particles. This is the nature of the problem. Now let us see what quantum mechanics predicts for the motion of the electron in such a situation.
 |
 |
 |
 |