An Introduction to the Electronic Structure of Atoms and Molecules
Professor of Chemistry / McMaster University / Hamilton, Ontario
An Introduction to the Electronic Structure of Atoms and MoleculesProfessor of Chemistry / McMaster University / Hamilton, Ontario
|
Listed below are a number of equations which give the dependence of ![]() ,
, ![]() and
and ![]() on
the quantum numbers n, l and m. They refer not only
to the hydrogen atom but also to any one-electron ion in general with a
nuclear charge of Z. Thus He+ is a one-electron ion with
Z
= 2, Li+2 another example with Z = 3.
on
the quantum numbers n, l and m. They refer not only
to the hydrogen atom but also to any one-electron ion in general with a
nuclear charge of Z. Thus He+ is a one-electron ion with
Z
= 2, Li+2 another example with Z = 3.
The average distance between the electron and the nucleus expressed
in atomic units of length is:
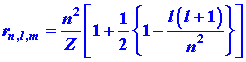 |
Note that ![]() is proportional to n2 for l =
0 orbitals, and deviates only slightly from this for l ¹
0. The value of
is proportional to n2 for l =
0 orbitals, and deviates only slightly from this for l ¹
0. The value of ![]() decreases
as Z increases because the nuclear attractive force is greater.
Thus
decreases
as Z increases because the nuclear attractive force is greater.
Thus ![]() for
He+ would be only one half as large as
for
He+ would be only one half as large as ![]() for H.
for H.
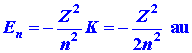 |
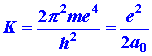 |
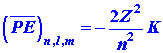 |
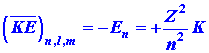 |
 |
 |
 |
 |